GSK Canada recently announced that Arexvy (respiratory syncytial virus (RSV) vaccine - recombinant, AS01E adjuvanted) was approved in Canada for preventing lower respiratory tract disease caused by RSV in individuals 60 and older.
Arexvy's availability in Canada is expected ahead of the 2023/24 peak RSV season, which is during the winter.
Previous RSV vaccine authorizations have been issued in Europe, the USA, and the U.K.
Marni Freeman, Country Medical Director, GSK, said in a press release on August 4, 2023, "With the approval of Arexvy, we are excited to be able to offer an option to help protect the nearly 10 million Canadians aged 60 and older who are at risk of RSV disease."
"We're hopeful that with a vaccine now available for older Canadians, the virus' burden on our healthcare system will also be dramatically improved."
"We look forward to working with provincial, territorial, and national health authorities to ensure older Canadians at greatest risk of RSV infection can access the vaccine."
RSV is a common, contagious virus that affects the lungs and respiratory airways. The virus can affect all ages, but the impact of RSV in older adults is significant.
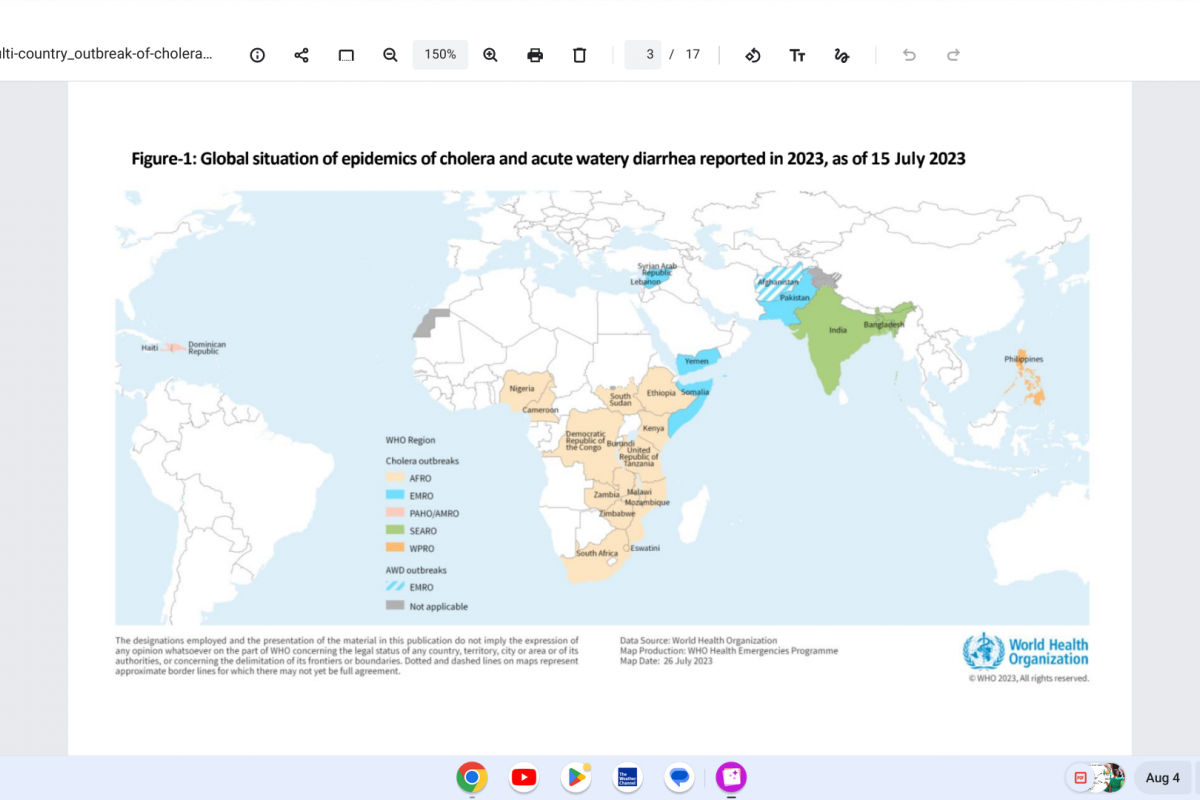
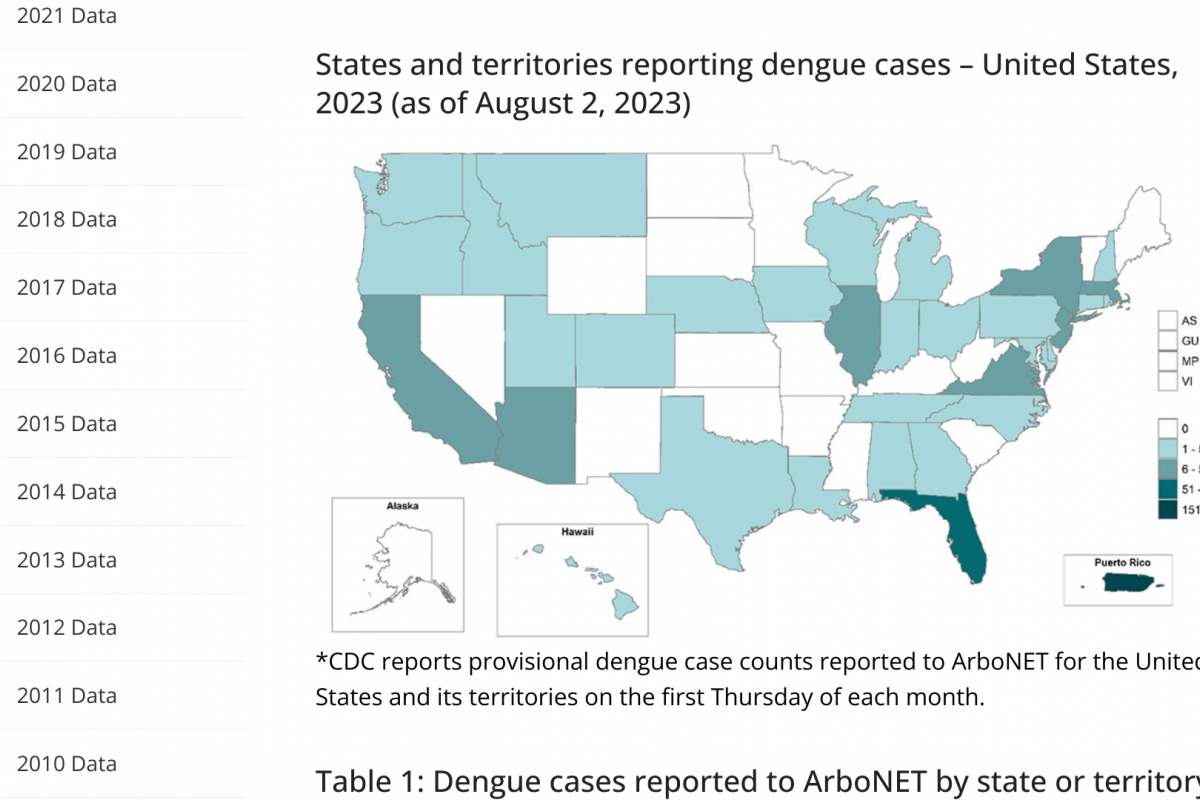
RSV is a seasonal respiratory virus generally identified first in Florida each year.