As the Northern Hemisphere heads into its fall respiratory season, most European countries are seeing an end to the recent COVID-19 wave.
Consultation rates of patients presenting to general practitioners with respiratory illness/acute respiratory infection increased in several countries in September 2023 but remained similar to the low levels observed in the same period last year.
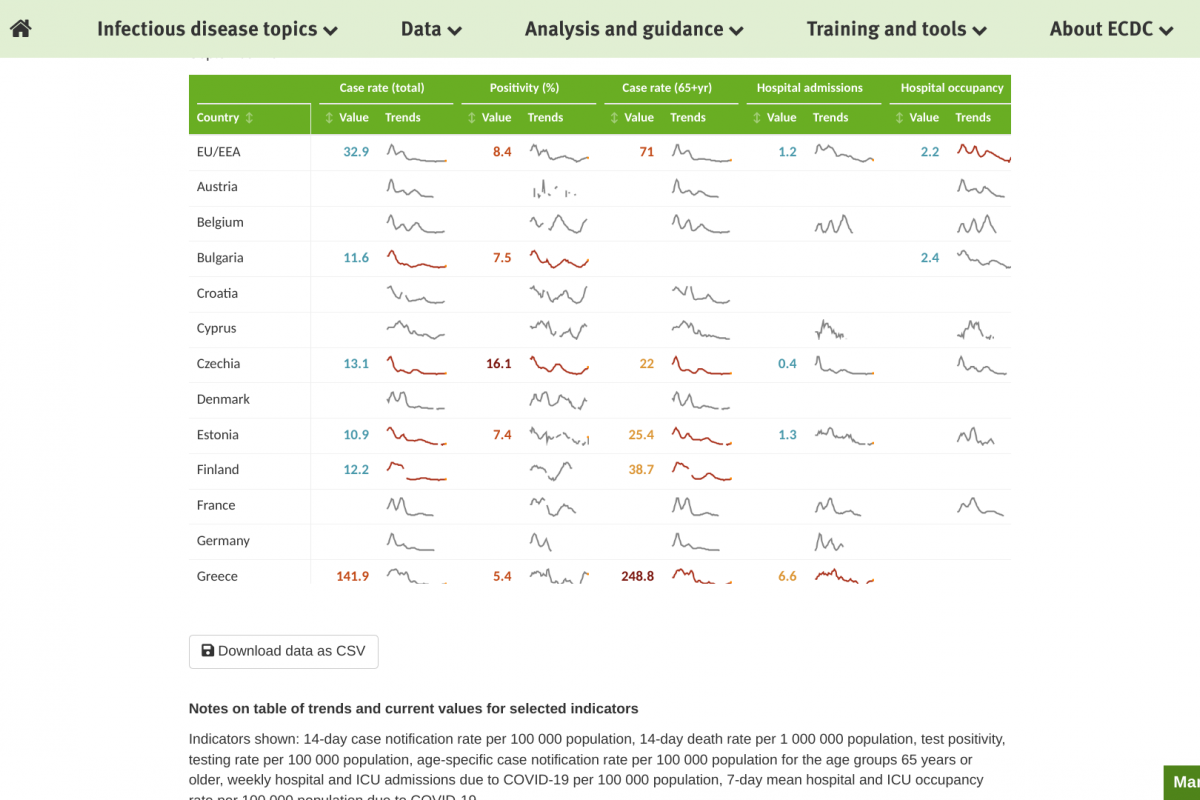
The European Centre for Disease Prevention and Control (ECDC) reported for week #36, there was a decrease in COVID-19 cases at the EU/EEA level, which is an inversion of the increasing trend observed in the previous weeks.
Of 19 countries reporting data as of September 14, 2023, twelve reported decreases in COVID-19 cases.
And the impact on severe disease and mortality remained limited in these European countries.
Of countries reporting COVID-19-related hospitalizations and deaths, one reported an increase in hospital admissions, and one reported an increase in fatalities.
Globally, as of September 13, 2023, the World Health Organization (WHO) reported there have been 770,563,467 confirmed cases of COVID-19, including 6,957,216 deaths since the pandemic began in late 2019.
As of September 5, 2023, a total of 13,500,135,157 vaccine doses have been administered.
The WHO has Listed twelve different COVID-19 vaccines during the pandemic.