Are Ebola Vaccine Boosters Needed Now
With an ample supply of World Health Organization-approved Zaire ebolavirus (EBOV) vaccines and antibody therapies available in 2024, a group of scientists recently presented a strategy to pre-empt the next outbreak.
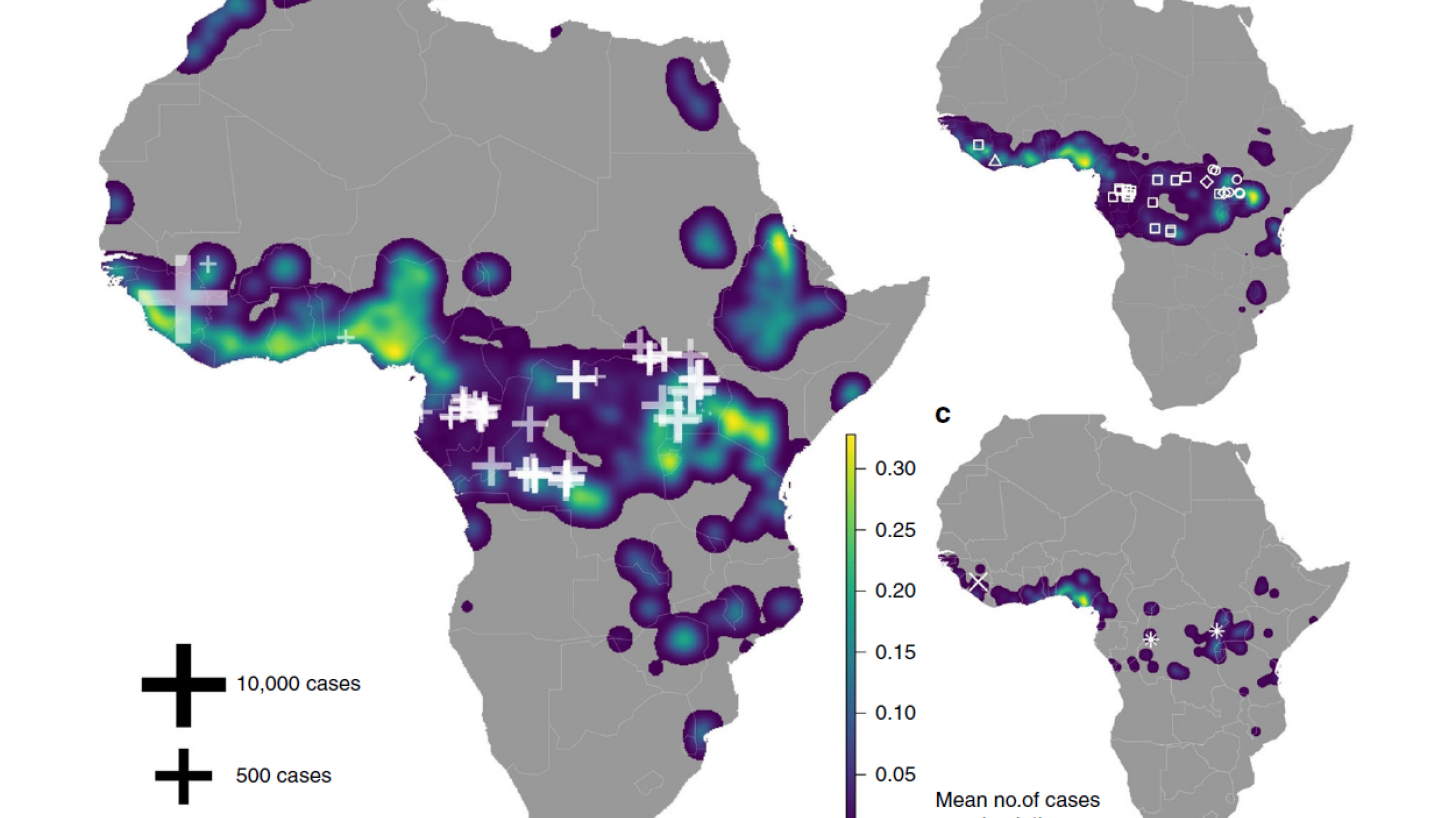
Ebolaviruses were first described in 1976 in the Democratic Republic of the Congo. Since then, ebola outbreaks have emerged periodically in several African countries.
The last Ebola outbreak was recorded in Uganda in 2022, resulting in 164 confirmed cases, including 55 deaths.
On May 8, 2024, scientists revealed that the number of individuals vaccinated against EBOV worldwide is estimated to range between 500,000 and 1,000,000.
They wrote that in the absence of long-term data on vaccine efficacy and duration of protection, we urgently need to understand revaccination strategies that could maximize the level of protection during the next outbreak.
In this Personal View article published by The Lancet Infectious Diseases, they highlighted the scarcity of available evidence to guide revaccination recommendations for the accumulating groups of previously vaccinated communities or front-line health-care workers that could be redeployed or re-exposed in the next EBOV outbreak(s).
This evidence base is crucial to identifying optimal target populations and the frequency of booster doses, guiding vaccine interchangeability, and preventing vaccine mistrust, equity concerns, and the exclusion of vulnerable populations.
They discussed five priority gaps (to whom, when, and how frequently to provide booster doses; long-term correlates and thresholds of protection; the effect of vector-directed immunity and viral variant protection; comparative research in mix-and-match schedules; and implementation concerns) that should be urgently tackled to adapt the initial EBOV prophylactic vaccination strategies considering potential booster dose vaccinations.
The complete, unedited article is posted at this link.
A recent U.S. Centers for Disease Control and Prevention (CDC) report disclosed that the International Coordinating Group (ICG) on Vaccine Provision has shipped 145,690 doses since 2021. Most vaccine doses (139,120; 95%) shipped from the ICG stockpile were repurposed for preventive vaccinations, compared with 6,570 (5%) used for outbreak response.
The CDC wrote on April 25, 2024, that repurposing Ebola vaccines for prevention could be prioritized to prevent transmission and maximize the cost-efficiency and benefits of the stockpile.
Previously, in 2022, the WHO recommended monoclonal antibody treatments that deliver passive immunization for Ebola infections: mAb114 (Ansuvimab; Ebanga) and REGN-EB3 (Inmazeb).
However, as of May 14, 2024, no approved vaccines protect people against the Sudan Ebolavirus (SUDV). The WHO has confirmed that SUDV candidate vaccines are being tested in the Solidarity Against Ebola human clinical studies.
Our Trust Standards: Medical Advisory Committee


