Promising HIV Vaccine Candidate Launches Phase 1 Clinical Trial
With over 40 million people living with HIV and more than 1 million new infections annually, the need for an effective preventive vaccine remains urgent.
On December 15, 2025, the first doses of experimental HIV vaccine antigens were administered at the Perinatal HIV Research Unit in Soweto, South Africa, marking the launch of the IAVI G004 Phase 1 clinical trial.
This trial advances the IAVI/Scripps Research strategy to train the immune system to produce broadly neutralizing antibodies (bnAbs) capable of protecting against diverse HIV strains. IAVI G004 builds on positive proof-of-concept data from earlier studies (G001, G002, and G003). It will evaluate three mRNA-based immunogens developed by IAVI and Scripps Research: eOD-GT8 60mer, Core-g28v2 60mer, and N332-GT5 gp151, delivered using Moderna's mRNA platform.
"It is very exciting to see the launch of the IAVI G004 clinical trial," said Mark Feinberg, M.D., Ph.D., President and CEO of IAVI, in a press release on January 6, 2026.
"We believe this is the most promising path toward an effective HIV vaccine."
Funding for the trial comes from the Bill & Melinda Gates Foundation, with materials manufactured by Moderna. The government of the Netherlands has provided additional support.
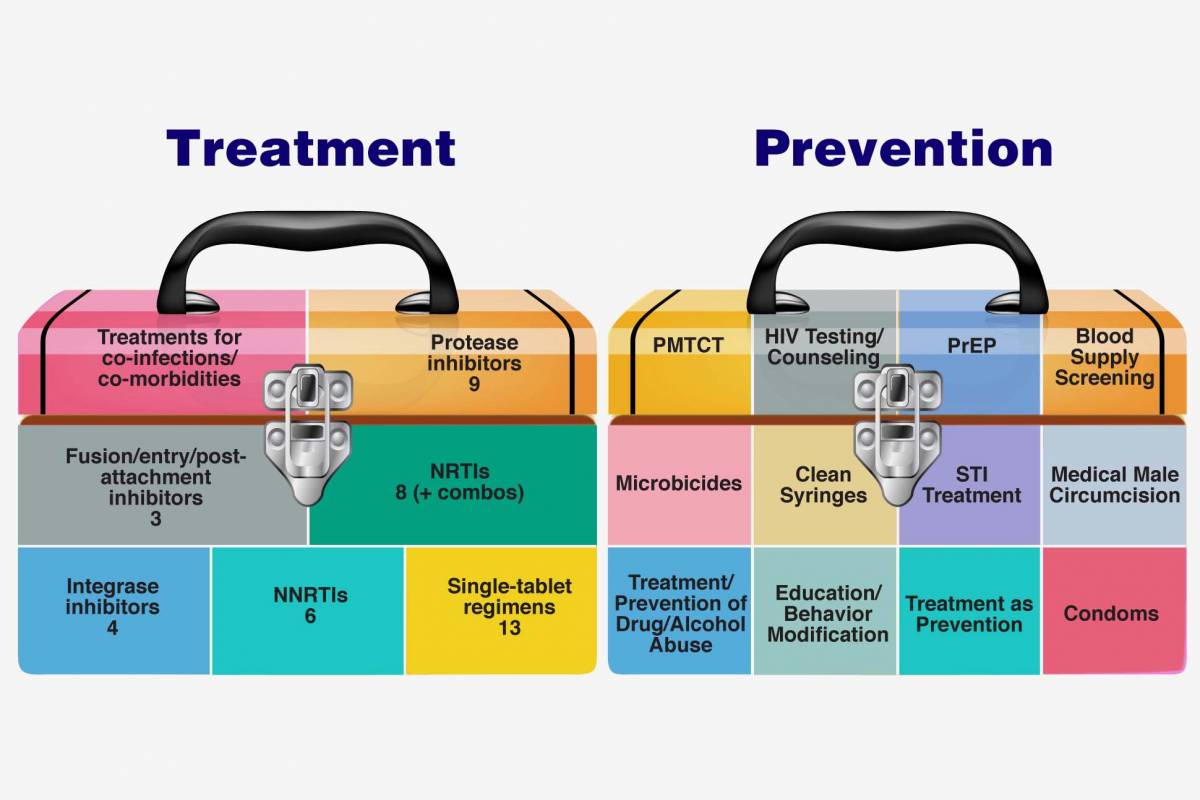
Currently, no HIV vaccine of any kind has been approved by the U.S. Food and Drug Administration (FDA), but scientists are actively pursuing new approaches. Therapeutic HIV vaccines are only available through a clinical trial.
The FDA says exploring therapeutic HIV vaccines to achieve HIV remission or a functional cure is essential, keeping viral load suppressed without the need for antiretroviral therapy. Ongoing HIV and AIDS clinical trials include studies of new HIV medicines, vaccines to prevent or treat HIV, and medicines to treat infections related to HIV and AIDS.
Our Trust Standards: Medical Advisory Committee