Lassa Fever Vaccine Candidate Advances in Clinical Study
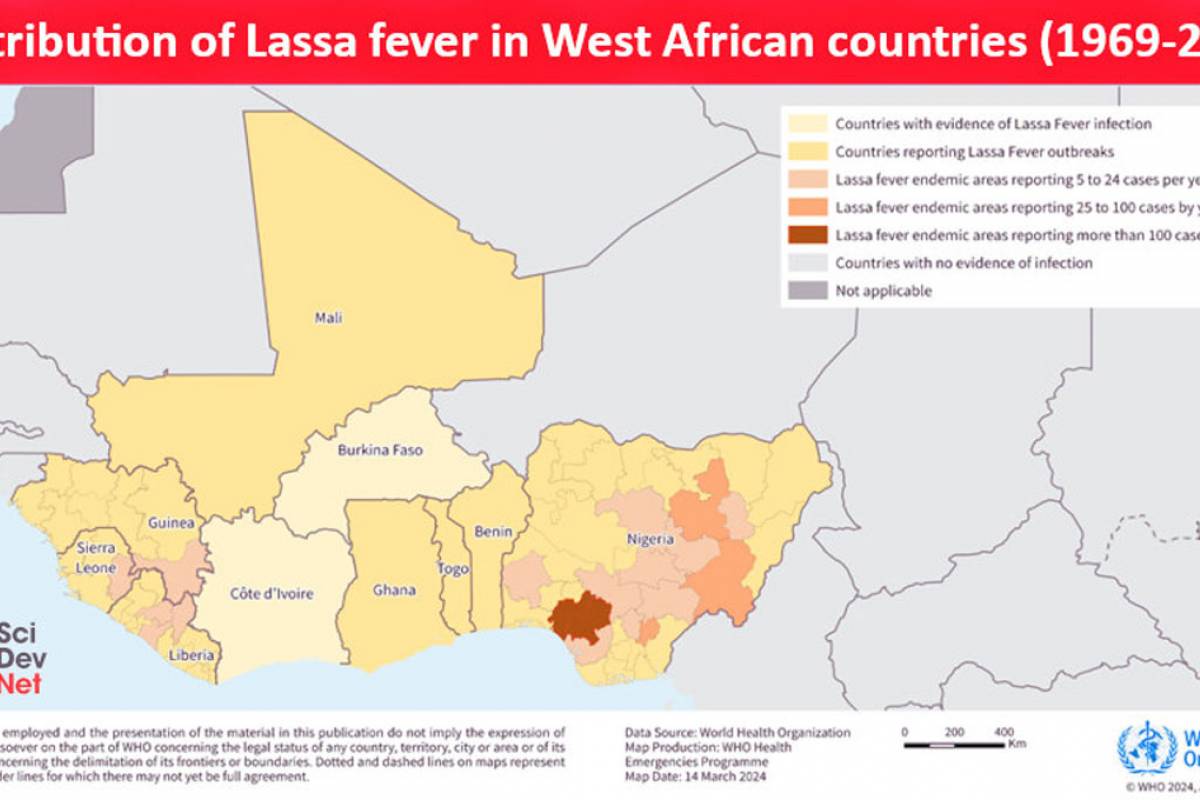
Currently, no vaccines or therapeutics are licensed against Lassa fever, an acute viral hemorrhagic illness caused by Lassa virus (LASV) that is responsible for thousands of deaths each year across West Africa, where the disease is endemic.
The findings from a first-in-human, Phase 1 clinical trial of IAVI's LASV vaccine candidate, published in the New England Journal of Medicine on November 6, 2025, demonstrate that one dose of the vaccine elicits robust and long-lasting immune responses and has an acceptable safety profile.
"Lassa fever is a cruel disease which has plagued West Africa for decades, including a deadly outbreak in Nigeria this year," said Dr. Kent Kester, Executive Director of Vaccine R&D at CEPI, in a press release.
"The promising Phase 1 data for IAVI's vaccine candidate takes us one step closer towards a much-needed Lassa fever vaccine, which, if successful, could save thousands of lives and avert millions of dollars of societal costs in the West African countries that bear the burden of this disease."
Our Trust Standards: Medical Advisory Committee

