$6.5 Million Funds S. aureus Vaccine Candidate Study
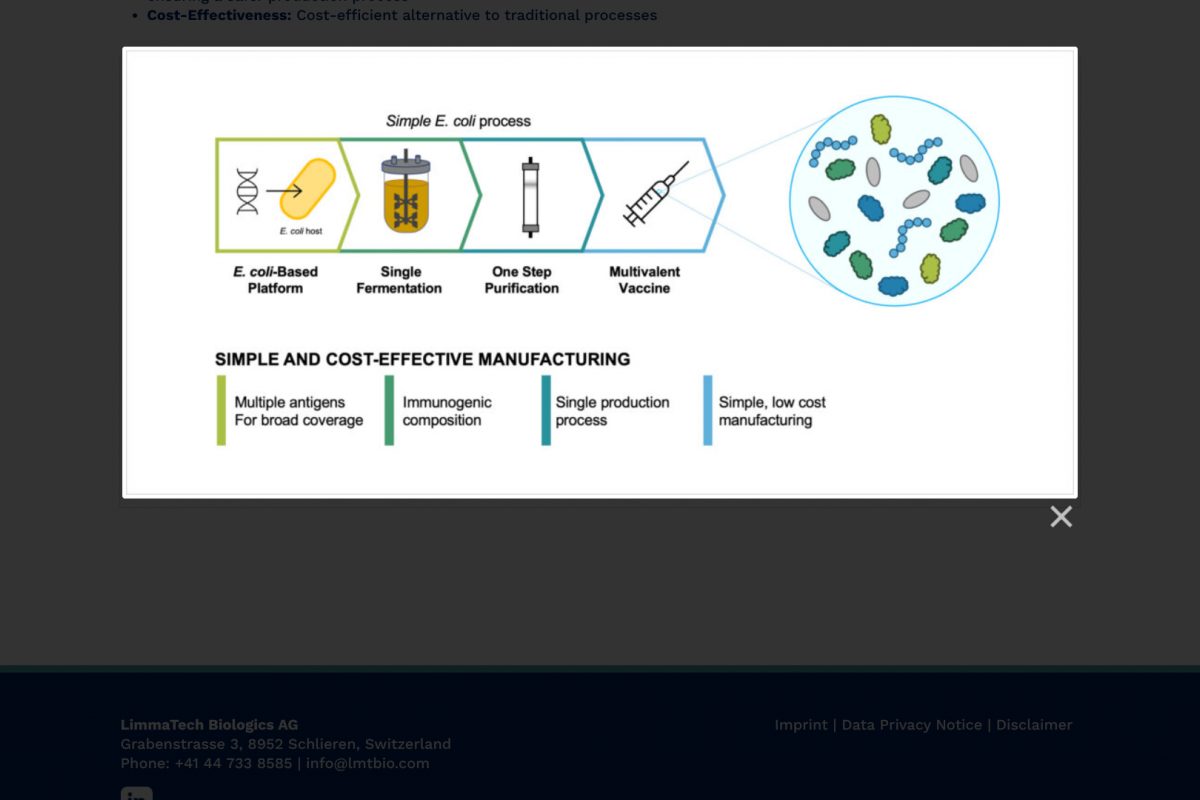
The first participants have been vaccinated in a Phase 1 clinical study of a multivalent vaccine candidate designed to prevent skin and soft tissue infections (SSTIs) caused by the bacterial pathogen Staphylococcus aureus (S. aureus).
S. aureus infections pose a significant global health challenge, causing an estimated 1 million deaths annually. Notably, 90% of all community-acquired S. aureus infections are SSTIs.
Furthermore, the World Health Organization has designated S. aureus a "high priority" pathogen, underscoring the urgency of developing innovative vaccine approaches and effective treatment strategies.
To address this essential health need, LimmaTech Biologics AG today announced that its LBT-SA7 vaccine candidate is expected to enroll 130 healthy adults aged 18-50 years, with initial results anticipated in the second half of 2025.
The company also announced the award of $6.5 million from the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) to advance the clinical development of LBT-SA7.
"Developing an S. aureus vaccine has long been a significant scientific challenge," explained Dr. Patricia Martin-Killias, Chief Operating Officer of LimmaTech, in a press release on February 17, 2025.
"We believe LBT-SA7 has the potential to provide a much-needed solution for those suffering from S. aureus infections. We are excited to launch the first-in-human clinical trial for LBT-SA7, bringing us closer to addressing an urgent global health challenge."
S. aureus is a Gram-positive bacterial pathogen that affects approximately 30% of the human population and causes a spectrum of infections, from SSTIs to severe conditions like pneumonia and bloodstream infections.
The company says traditional antibiotic treatments, both oral therapy and intravenous administration reserved for severe cases, have become increasingly less effective due to the rise of antibiotic resistance.
Federal funds from the U.S. Department of Health and Human Services partly fund CARB-X's funding for this project.
Our Trust Standards: Medical Advisory Committee
