China Approves HPV Vaccine for Young Men

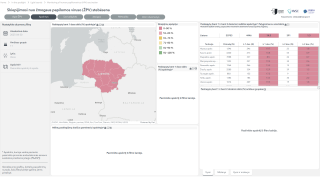
Merck today announced China's National Medical Products Administration approved GARDASIL® Human Papillomavirus Vaccine (HPV) for use in males 9-26 years of age to help prevent certain HPV-related cancers and diseases.
This approval makes GARDASIL the first HPV vaccine approved for use in males in China as of January 8, 2025.
GARDASIL is now indicated in China to prevent anal cancers caused by HPV Types 16 and 18, genital warts (condyloma acuminata) caused by HPV Types 6 and 11, and the following precancerous or dysplastic lesions caused by HPV Types 6, 11, 16, and 18: grade 1, grade 2, and grade 3 anal intraepithelial neoplasia.
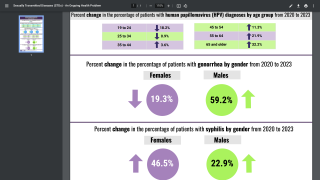
Joseph Romanelli, president, Human Health International, Merck, commented in a press release, “Since first approval, our HPV vaccines have helped protect over 50 million females in China from certain HPV-related cancers and diseases. With this expanded approval, we look forward to helping protect this new population of Chinese males from certain HPV-related cancers and diseases.”
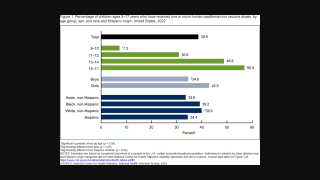
Gardasil and other HPV vaccines will be in use by various countries in 2025.
Our Trust Standards: Medical Advisory Committee