Investigational Autologous Vaccine Therapy Receives Clearance for a First in Human Clinical Study for Stage III/IV Ovarian Cancer Treatment
PhotonPharma, a biotechnology company that aims to revolutionize cancer treatment, announced today that it has received clearance from the U.S. Food and Drug Administration to proceed with its Phase I clinical study for the treatment of Stage III/IV ovarian cancer.
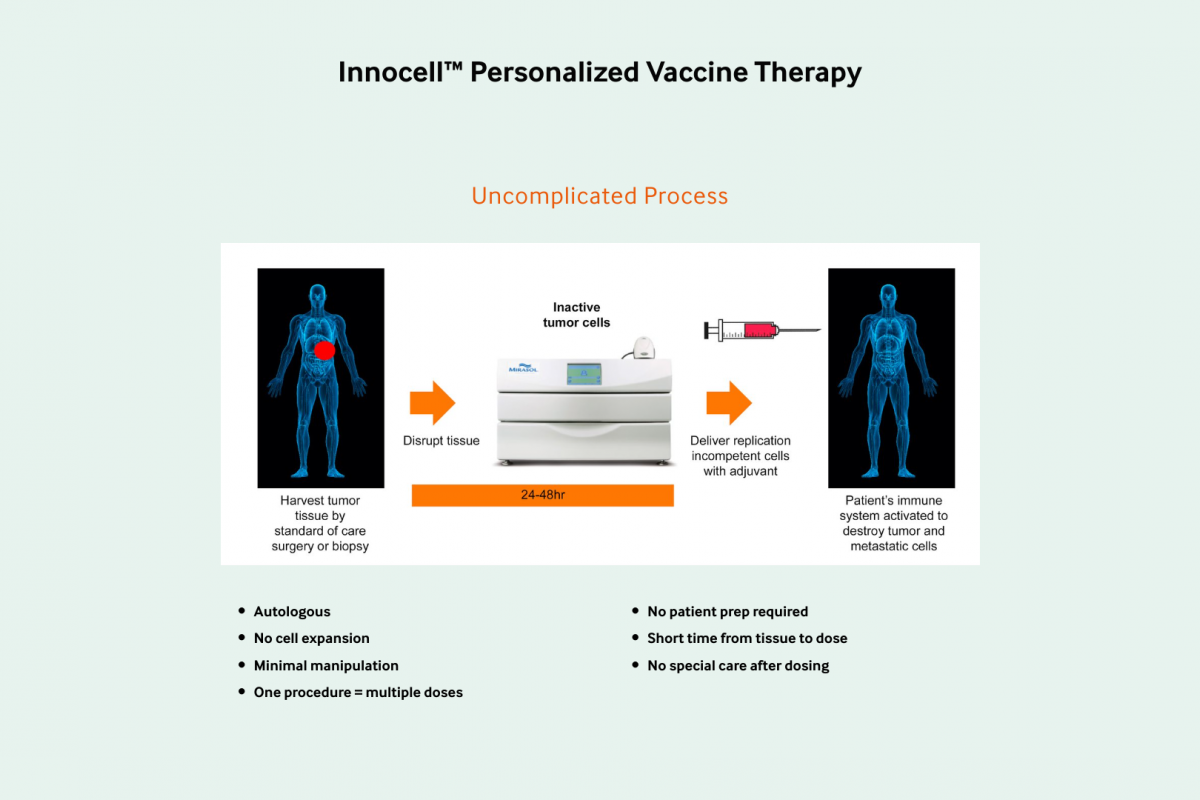
The company will use Innocell™, its groundbreaking investigational autologous cell-based vaccine therapy, for this purpose. The vaccine therapy will be manufactured at City of Hope's Los Angeles campus.
This therapy is based on the use of inactivated tumor cells prepared with a proprietary process that involves UV light and riboflavin.
These cells are isolated from a patient's tumor and inactivated and then used in a treatment that is designed to stimulate the patient's immune system to fight cancer.
Alan Rudolph, the CEO of PhotonPharma, expressed his enthusiasm about this development in a press release on March 5, 2024, stating, "We are thrilled to have reached this pivotal moment in our journey toward providing a novel treatment option for patients facing advanced ovarian cancer."
PhotonPharma anticipates initiating patient enrollment for this study shortly to profile the therapeutic potential of this innovative autologous vaccine therapy.
Our Trust Standards: Medical Advisory Committee