Mpox Vaccine Candidate Obtains Project NextGen Funding

Tonix Pharmaceuticals Holding Corp. today announced that the National Institute of Allergy and Infectious Diseases (NIAID) will conduct a Phase 1 clinical trial with TNX-1800 (recombinant horsepox virus, live vaccine), expected to start in the second half of 2024.
The Phase 1 study involving TNX-1800 assesses safety and immunogenicity in approximately 60 healthy adult volunteers.
Tonix is developing a novel vaccine platform that, primarily by eliciting a T-cell immune response, will provide durable protection against severe disease and prevent forward transmission.
A related horsebox-based vaccine, TNX-8011, protected animals against challenge with monkeypox virus delivered directly into the lungs.
TNX-801 is also the vector on which TNX-1800 is based and has been shown to be >1,000-fold more attenuated than modern vaccinia virus vaccine (VACV) strains in immunocompromised mice.
Seth Lederman, M.D., CEO of Tonix Pharmaceuticals, commented in a press release on November 2, 2023, "TNX-1800 will be the first vaccine candidate using our live virus recombinant pox virus (RPV) platform technology to enter clinical trials."
"We hope to expand the portfolio of RPV-based vaccines to address several other known respiratory threats, including smallpox, mpox and tuberculosis."
"We are committed to supporting NIAID in assembling a variety of vaccine platform options to ensure the availability of effective vaccines in the face of known and emerging threats."
"We look forward to participating in the Project NextGen initiative."
Project NextGen is an initiative by the U.S. Department of Health and Human Services to advance a pipeline of new, innovative vaccines and therapeutics.
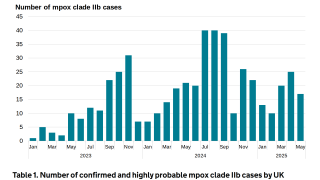
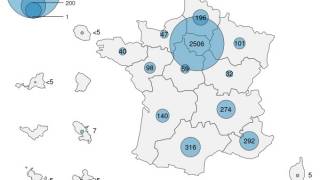
As of November 2023, mpox vaccines are U.S. FDA-approved and available in certain cities.
Our Trust Standards: Medical Advisory Committee