First mRNA Malaria Vaccine Candidate Launches Clinical Trial

BioNTech SE today announced the initiation of a first-in-human, dose escalation, Phase 1 study with BNT165b1, the first candidate from the Company’s BNT165 program, to develop a multi-antigen malaria vaccine candidate.
BioNTech will initially evaluate a set of mRNA-encoded antigens of the malaria-causing parasite Plasmodium falciparum (P. falciparum) to help select the multi-antigen vaccine candidate to proceed to planned later-stage trials.
This first clinical trial (last updated on October 14, 2022) will evaluate the safety, tolerability, and exploratory immunogenicity of the vaccine candidate BNT165b1 at three dose levels.
BioNTech’s placebo-controlled, observer-blinded Phase 1 trial is expected to enroll approximately 60 healthy volunteers with no history of previous or current malaria infection throughout several sites in the U.S.
“Our objective is to develop a vaccine that can help to prevent Malaria and reduce mortality. Over the next months, we aim to evaluate different antigens with scientific rigor to identify the optimal candidate,” said Prof. Özlem Türeci, M.D., Chief Medical Officer and Co-Founder of BioNTech, in a press release on December 23, 2022.
Malaria is an infectious disease caused by Plasmodium parasites.
The parasite is transmitted to humans mainly through the bites of infected Anopheles mosquitos, where it enters the human bloodstream and travels to the liver to mature and divide.
After the release from the liver cells, the parasite re-enters the bloodstream, where it multiplies rapidly and can cause disease and death.
Moreover, it can be taken up again by a feeding mosquito.
The symptoms of malaria infection in humans are high fever, vomiting, and other flu-like symptoms, according to the U.S. Centers for Disease Control and Prevention.
If untreated or treated late, severe infections have a mortality rate of up to 50%.
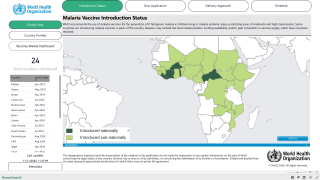
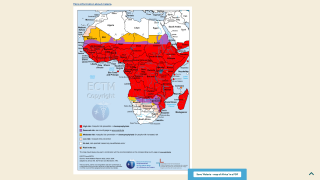
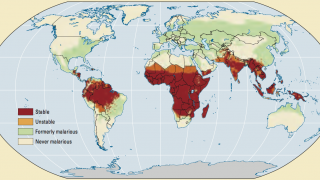
The disease is widespread in tropical and subtropical regions, with 95% of all cases occurring in Africa, with children under five years old and pregnant women being the most vulnerable population.
As of today, the U.S. Food and Drug Administration has not approved a malaria vaccine.
However, two malaria vaccines are currently authorized in various countries.
Other malaria vaccine news is posted at Vax-Before-Travel.com/Malaria.
Our Trust Standards: Medical Advisory Committee