Approved Malaria Vaccine Awarded WHO Prequalification
London-based GSK plc's Thomas Breuer, Chief Global Health Officer, commented yesterday that the “World Health Organization (WHO) prequalification of Mosquirix™ (RTS,S) is a key step in reaching children with the first and only approved malaria vaccine."
"Malaria remains a significant cause of illness and death for children in many parts of the world, and it is a significant driver of inequality."
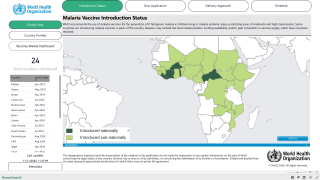
"So far, over one million children in Ghana, Kenya, and Malawi have had at least one dose of Mosquirix."
The "WHO’s prequalification paves the way for more children to benefit from the (malaria) vaccine," stated Breuer in a related press release on September 6, 2022.
Mosquirix vaccination triggers the human immune system to defend against the first stages of disease when the Plasmodium falciparum malaria parasite enters the host's bloodstream through a mosquito bite and infects liver cells.
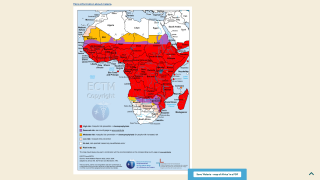
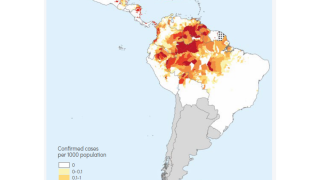
On October 6, 2021, the WHO recommended the RTS,S malaria vaccine for sub-Saharan Africa and regions with moderate to high malaria transmission.
GSK is currently working with partners to accelerate a product transfer for long-term antigen production, including technology transfer, to Bharat Biotech of India.
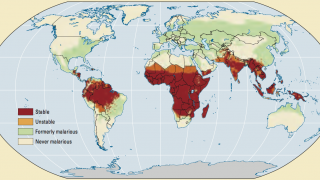
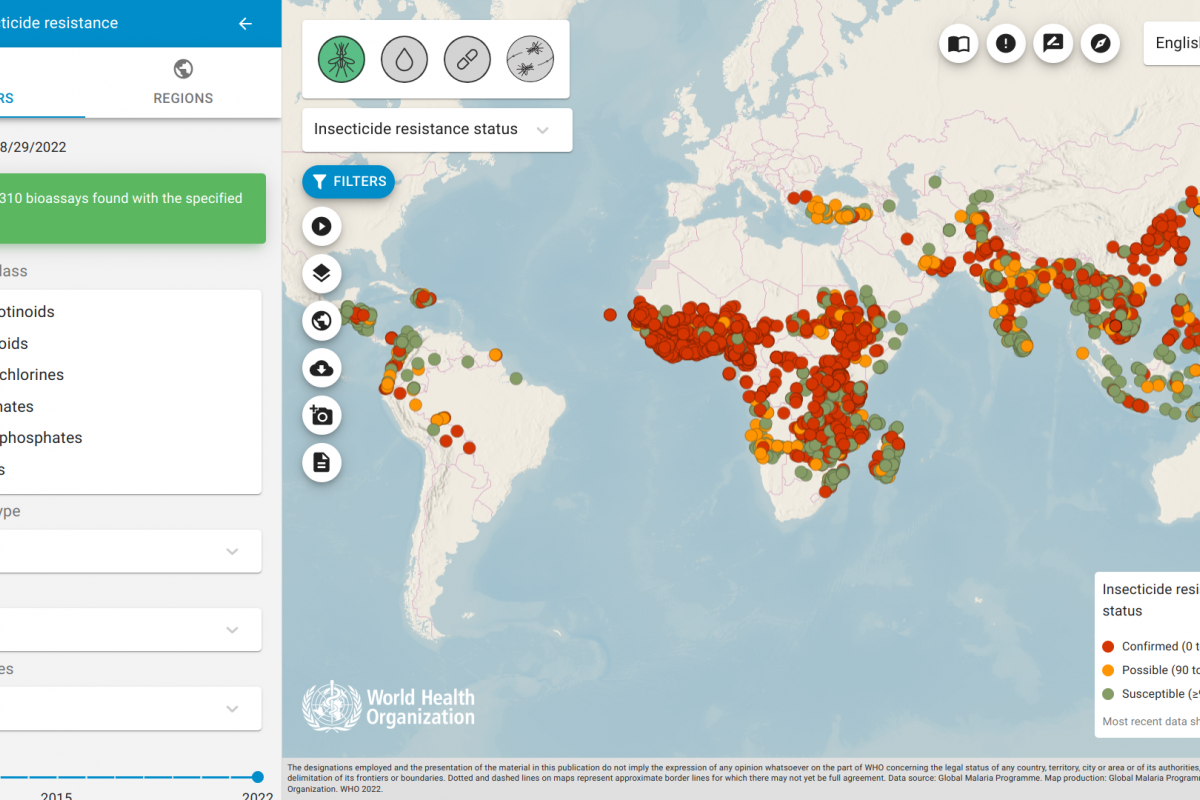
According to the U.S. CDC, Malaria is a vaccine-preventable mosquito-borne disease caused by a parasite.
As of September 7, 2022, the U.S. FDA had not approved a malaria vaccine.
Additional malaria vaccine news is posted at Vax-Before-Travel.com/Malaria.
Our Trust Standards: Medical Advisory Committee