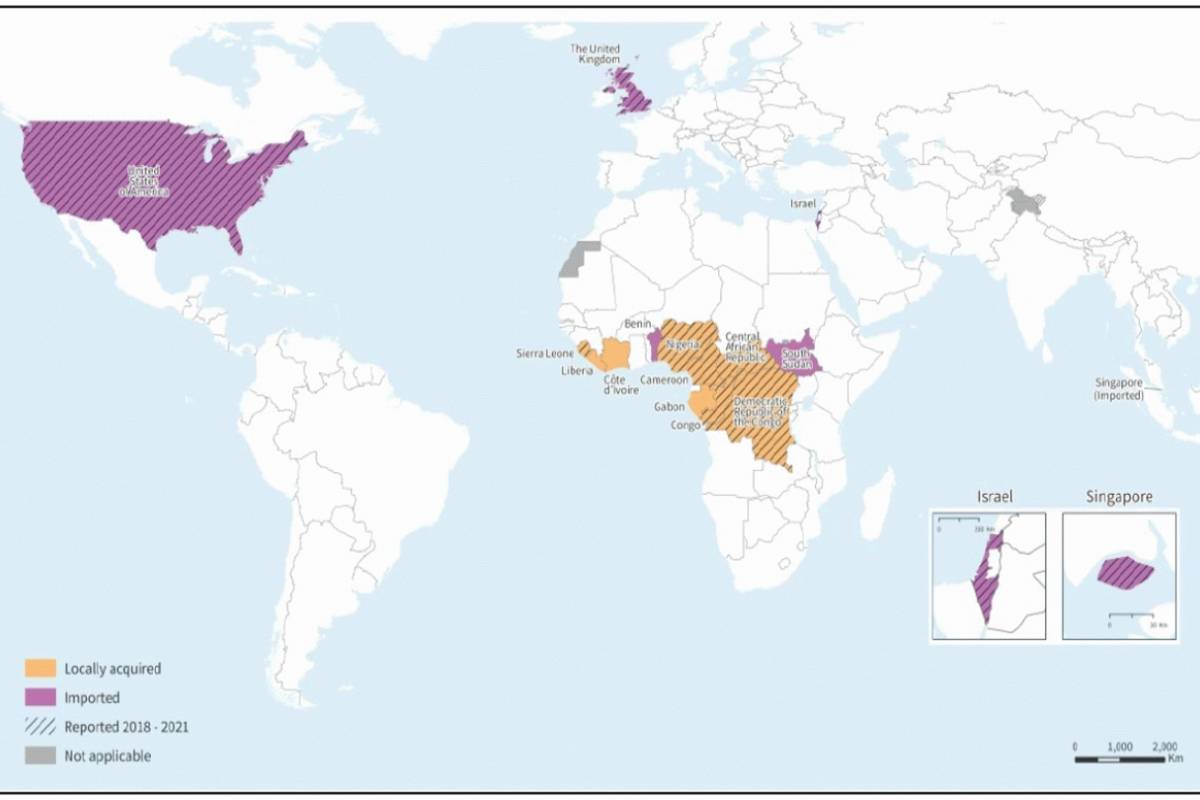
Tonix Pharmaceuticals Holding Corp. and Bilthoven Biologicals (BBio) today announced a collaboration to advance TNX-801, a mpox vaccine candidate.
TNX-801 (recombinant horsepox virus) is a live replicating, attenuated, single-dose vaccine candidate based on horsepox in preclinical development to prevent mpox and smallpox.
TNX-801 is based on technology that has the potential to be used as a viral vector platform from which recombinant versions can be developed to protect against other infectious diseases.
BBio is a global vaccine company that produces prophylactic and therapeutic vaccines and is part of the world’s largest vaccine manufacturer, the Cyrus Poonawalla Group, which includes the Serum Institute of India.
BBio has been selected by the European Union for its pandemic preparedness program of ‘ever warm’ vaccine manufacturing companies.
“The recent mpox outbreak exemplifies precisely why we built the pandemic preparedness facility at BBio,” said Jurgen Kwik, Chief Executive Officer of Bilthoven Biologicals, in a press release on August 26, 2024.
“The establishment of the 'ever-warm' facility for pandemic preparedness underscores the critical importance of readiness in the face of global health emergencies, such as mpox. This collaboration encapsulates the essential role of the facility in bolstering pandemic preparedness and response capabilities."
Tonix has received an official written response from a Type B pre-Investigational New Drug Application meeting with the U.S. Food and Drug Administration to develop TNX-801 as a potential vaccine to protect against mpox and smallpox diseases.
Currently, four mpox / smallpox vaccines are in use globally.