New research by modeling experts shows that vaccinating against Lassa Fever—a viral disease—would prevent millions of people from falling ill.
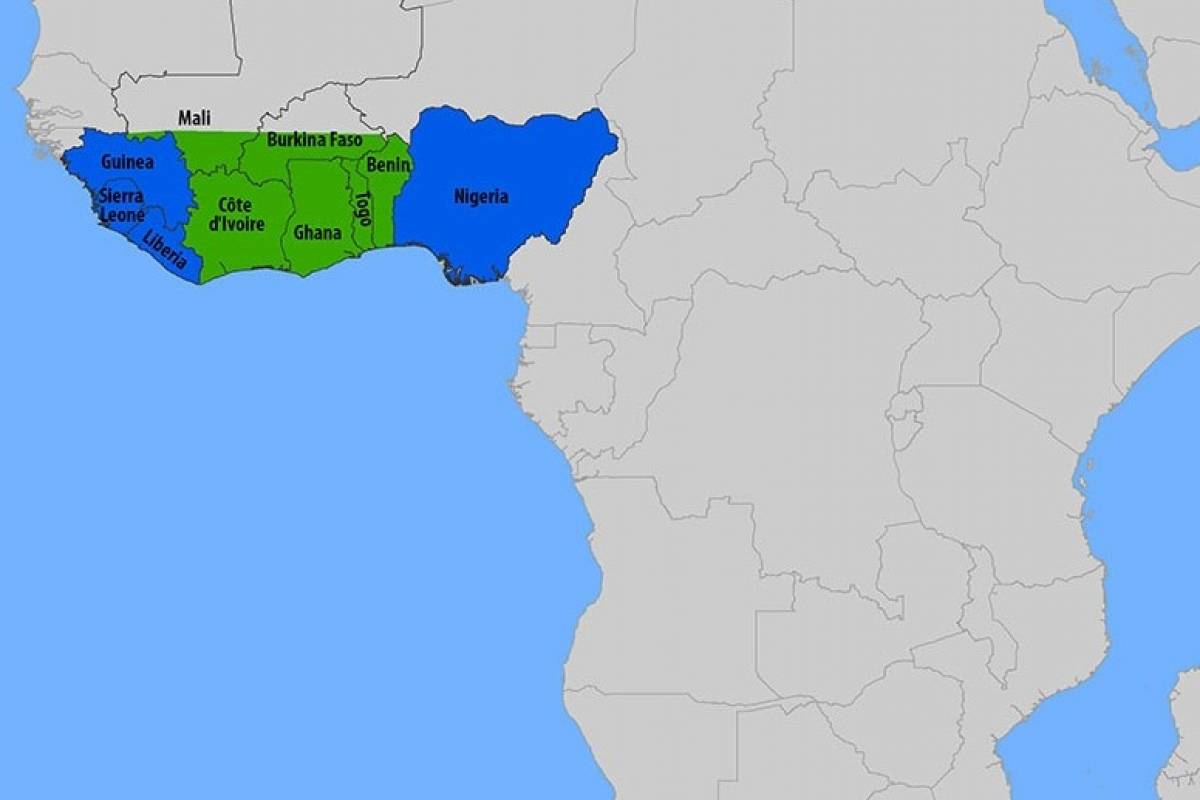
Lassa fever is found in parts of West Africa, including Sierra Leone, Liberia, Guinea, and Nigeria, where the first documented case occurred in 1969.
According to modeling by the Universities of Oxford and Liverpool and the Liverpool School of Tropical Medicine, deploying an effective Lassa vaccine across 15 countries of continental West Africa could save nearly 3,300 lives over ten years, wrote the Coalition for Epidemic Preparedness Innovations (CEPI).
The model was published on August 28, 2024, and predicted 2.7 million (95% uncertainty interval: 2.1–3.4 million) Lassa virus infections annually. However, due to limited access to diagnostics and healthcare, Lassa’s true disease burden could be much higher than reported.
They also model the emergence of ‘Lassa-X’—a hypothetical pandemic Lassa virus variant—and project impacts of achieving 100 Days Mission vaccination targets.
The results showed that around 5,500 lives could be saved and 33,000 hospitalizations avoided throughout a two-year outbreak if safe and 70% effective Lassa X vaccines were given to 40% of people per year starting within 100 days.
Overall, the most effective vaccination strategy was a population-wide preventive campaign targeting WHO-classified ‘endemic’ districts.
Richard Hatchett, CEO of CEPI, commented in a press release, “This study demonstrates the urgent need for a vaccine to protect people from this debilitating and sometimes deadly disease. Lassa fever has been a priority for CEPI since our launch in 2017, and we are proud to be one of the world’s leading Lassa vaccine R&D funders.”
CEPI is one of the world’s leading Lassa vaccine candidate R&D funders. To date, it has invested in six potential vaccine candidates, of which four have progressed into human testing.
One of CEPI’s partners, IAVI, has launched the first-ever Phase II clinical trial of a Lassa vaccine in Abuja, Nigeria.