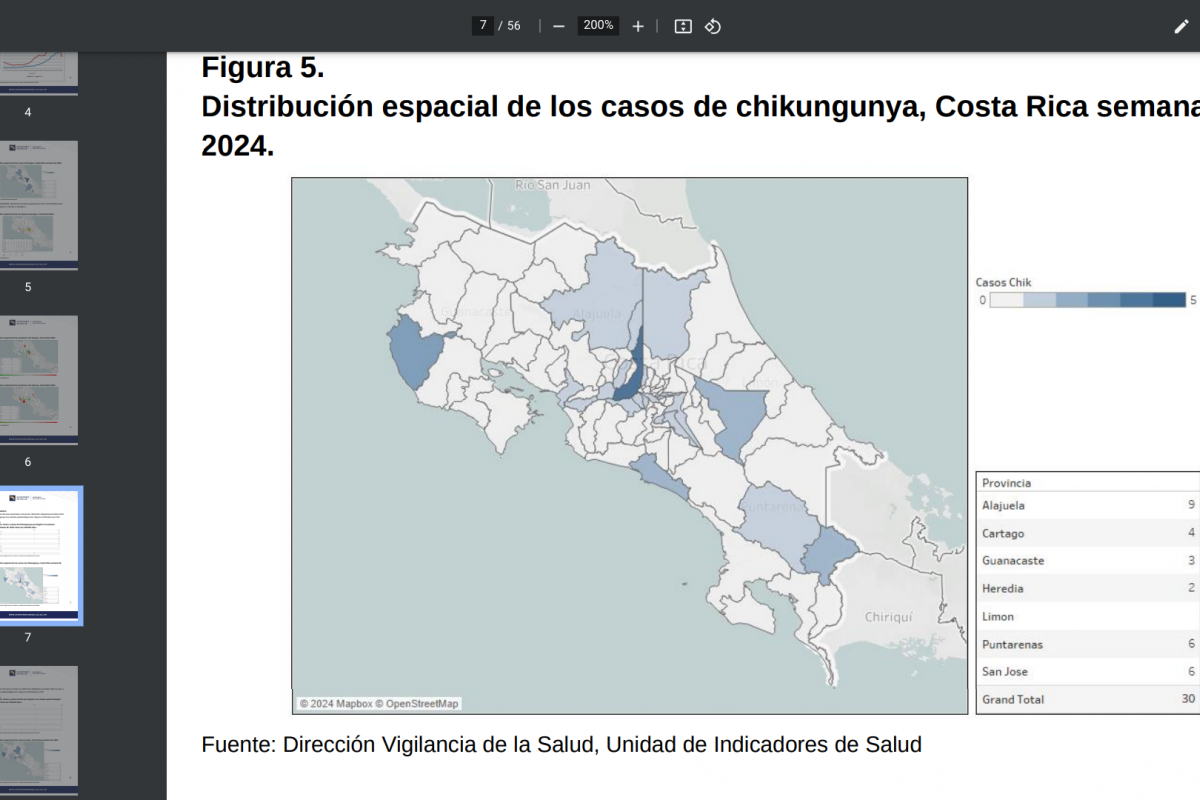
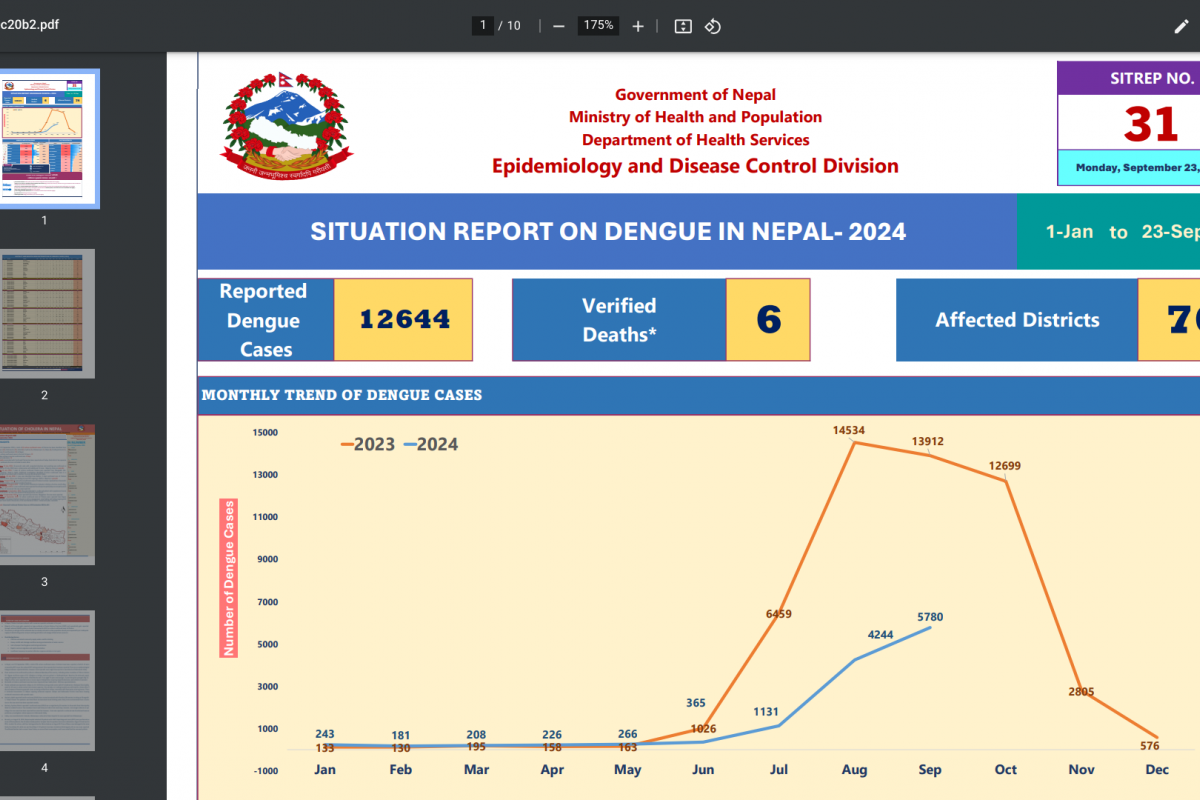
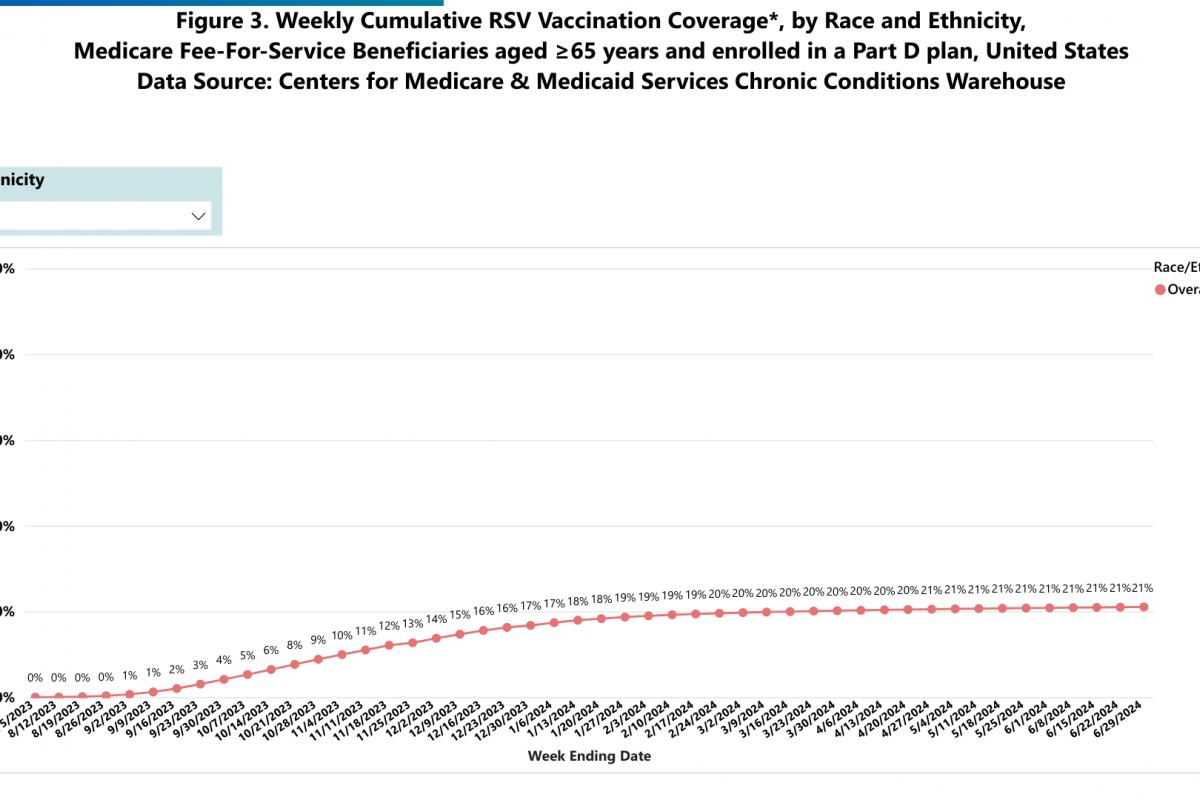
The World Health Organization (WHO) today announced its influenza vaccine strain recommendations for the Southern Hemisphere's 2025 flu season. The WHO makes these recommendations twice yearly, once for each hemisphere.
The WHO recommends manufacturers produce trivalent (three-strain) vaccines for the Southern Hemisphere's upcoming flu season.
"The periodic update of viruses contained in influenza vaccines is necessary for the vaccines to be effective due to the constantly evolving nature of influenza viruses, including those circulating and infecting humans," the WHO wrote on September 27, 2024.
From February through August 2024, the WHO reported influenza activity was detected in all transmission zones. Overall, detections were higher compared to the same reporting period in 2023, primarily due to higher detections in the Americas.
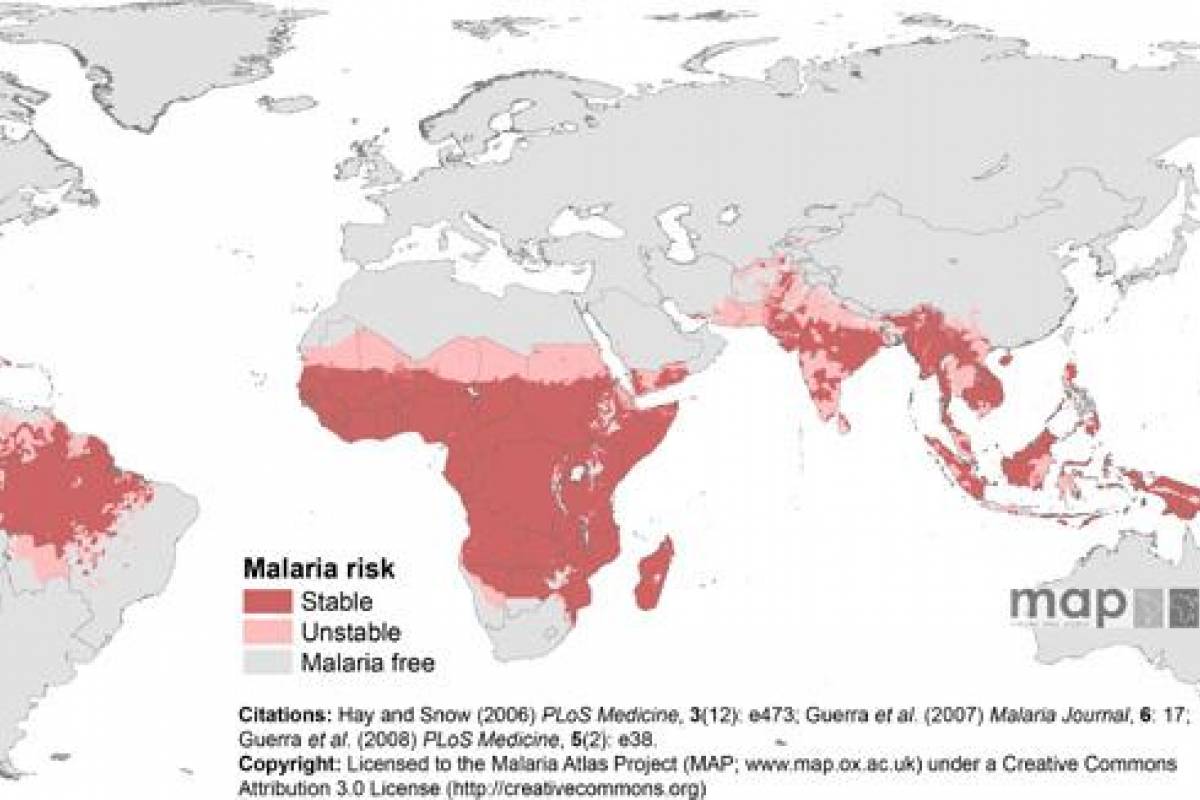
According to Google Maps, the Southern Hemisphere is the half of the Earth below the equator. It includes Antarctica, Australia, and parts of Africa, Asia, and South America.
As of September 2024, the flu season in the United States has been reported as mild, and millions of flu shots have already been delivered to local health clinics and pharmacies.