ADvantage Therapeutics, Inc. today announced that the first patient was enrolled in the Phase 2b clinical trial on its lead immunotherapy candidate, AD04™, for treating mild Alzheimer's disease (AD).
The study is authorized to be conducted in Austria, France, Poland, Bulgaria, and Slovakia and is expected to expand to Germany and the U.K. in the coming months.
AD04 has been used as an adjuvant in human and animal vaccination programs.
In a previous trial, AD04 serving as a control against another compound demonstrated a statistically significantly slower decline over other treatment groups in cognitive and quality-life clinical measures.
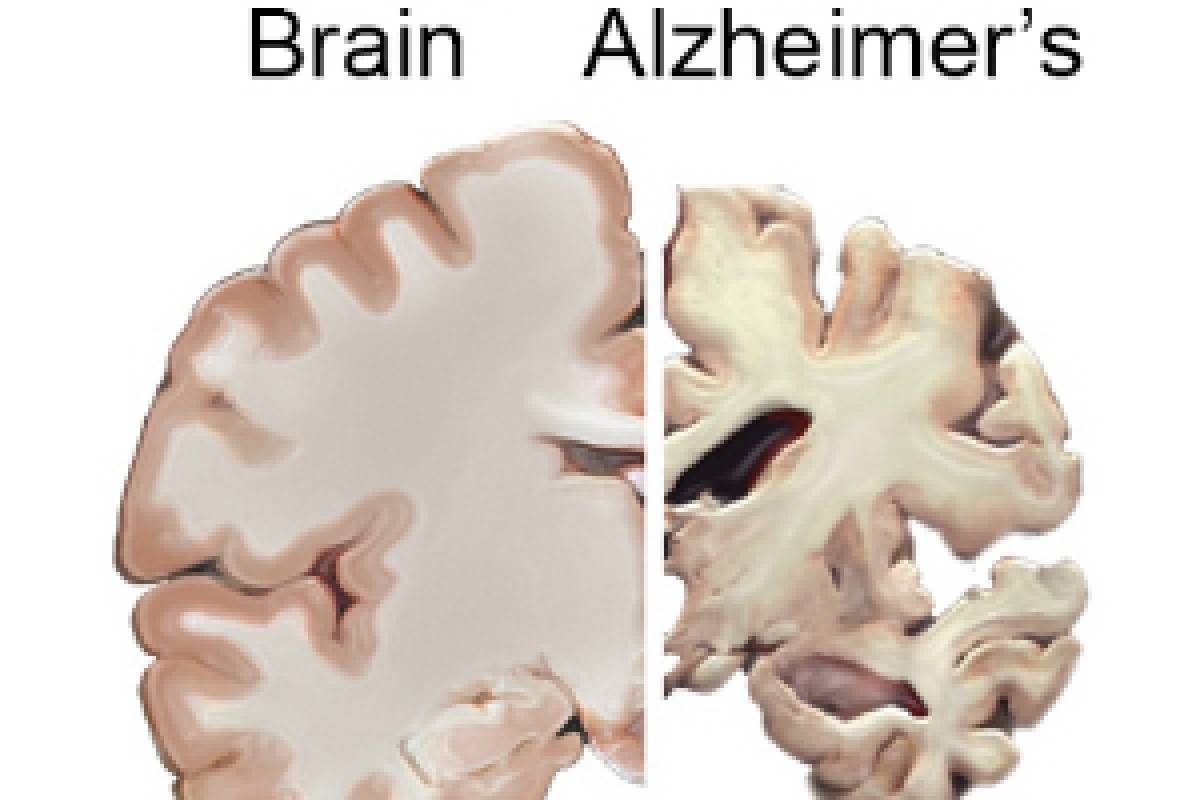
AD04 also showed a slower decline in hippocampal volume as a biomarker.
The Company believes that rather than being limited to a specific aspect of AD pathology, such as amyloid beta or tau, AD04 may address immunological mechanisms in the brain. And AD04 may function as an immunomodulator, stimulating and/or regulating the immune system to reduce AD pathology.
Dr. Andreas Winkler, MSc., principal investigator at Institut Neuromed, commented in a press release on November 27, 2023, "... AD04 represents a significant departure from approaches seen to date and one that may change our understanding of how to manage it as well as the disease itself."
Scientists do not yet fully understand what causes Alzheimer’s disease. Alzheimer’s is the most common type of dementia. About forty-four million people worldwide have Alzheimer's Disease, and it is the sixth leading cause of death in industrialized countries. The socio-economic burden of AD is enormous, says the U.S. CDC.
“We consider AD an autoimmune disease,” said Jeffrey Madden, CEO of ADvantage, in May 2023.
“And, instead of going after the consequences of AD, such as tau-protein and amyloid aggregates, we analyze immunological and neuroinflammatory events in specific parts of the brain. We believe these are causes of brain shrinkage and cognitive decline seen in AD.”
As of November 27, 2023, the U.S. FDA, the European Medicines Agency, and the U.K. have not approved an AD preventive vaccine candidate.