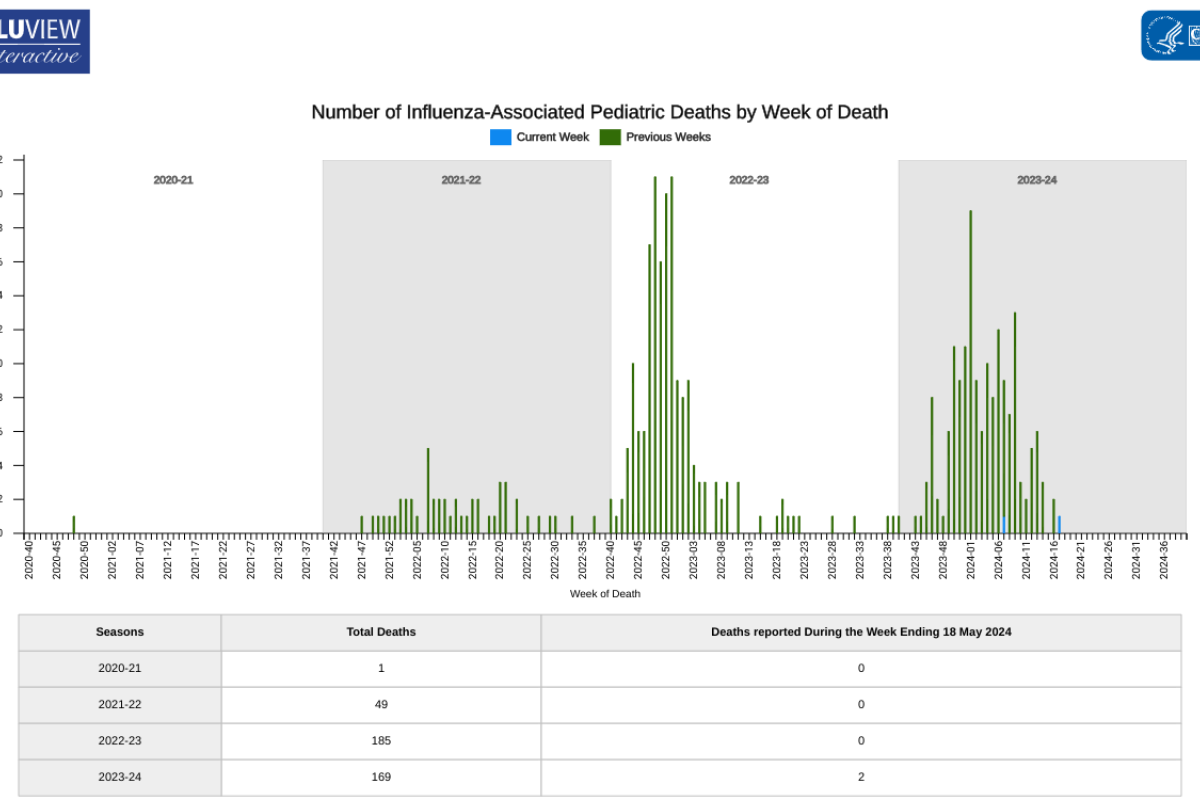
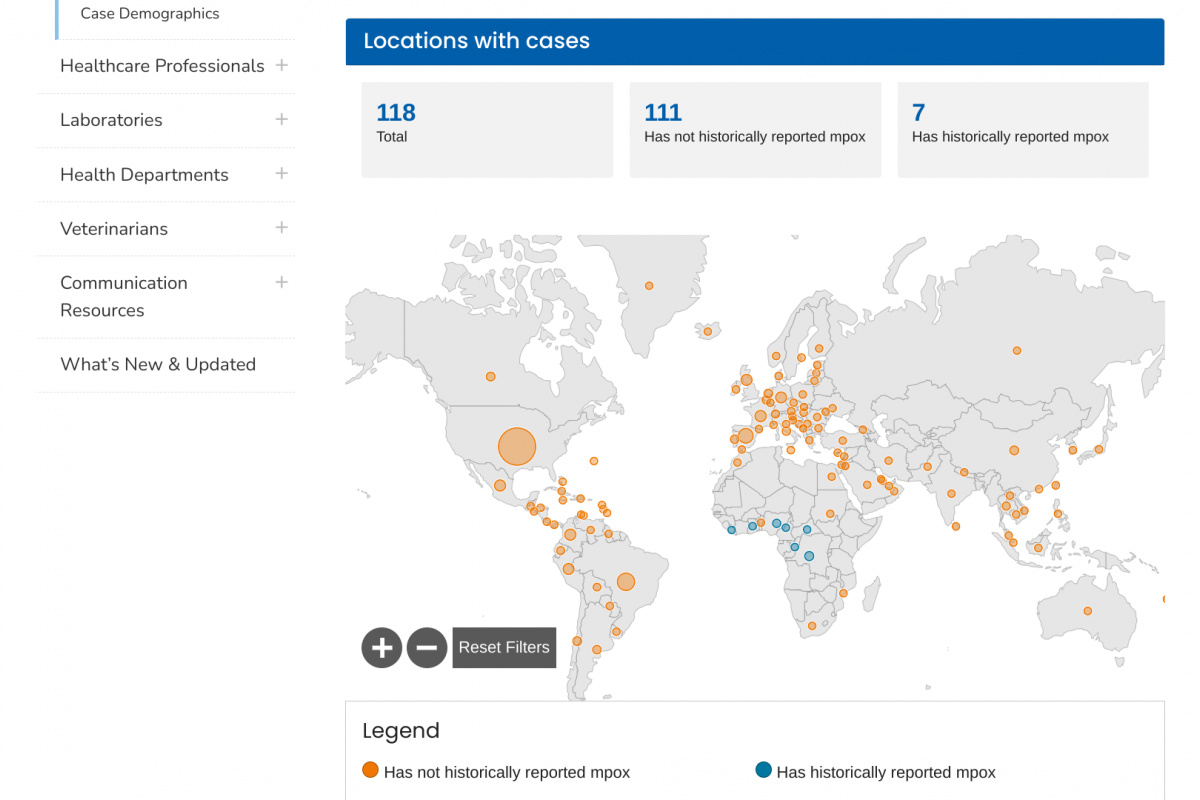
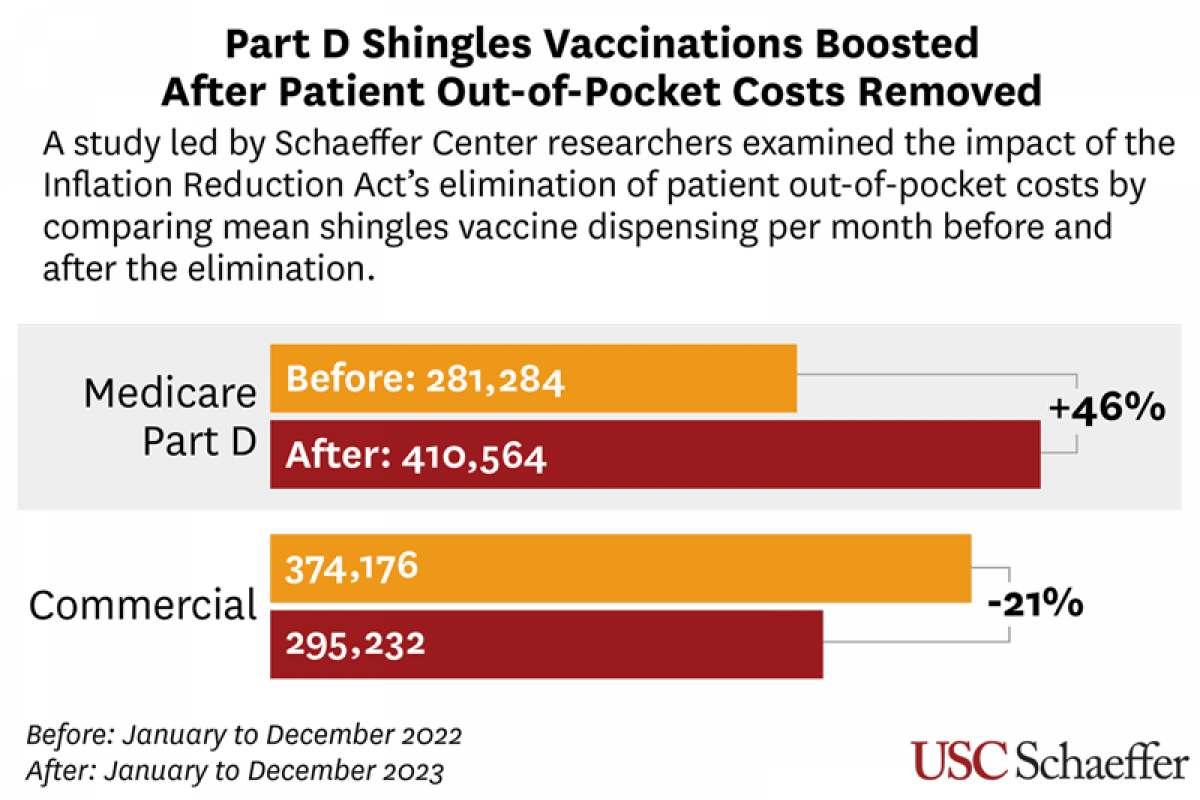
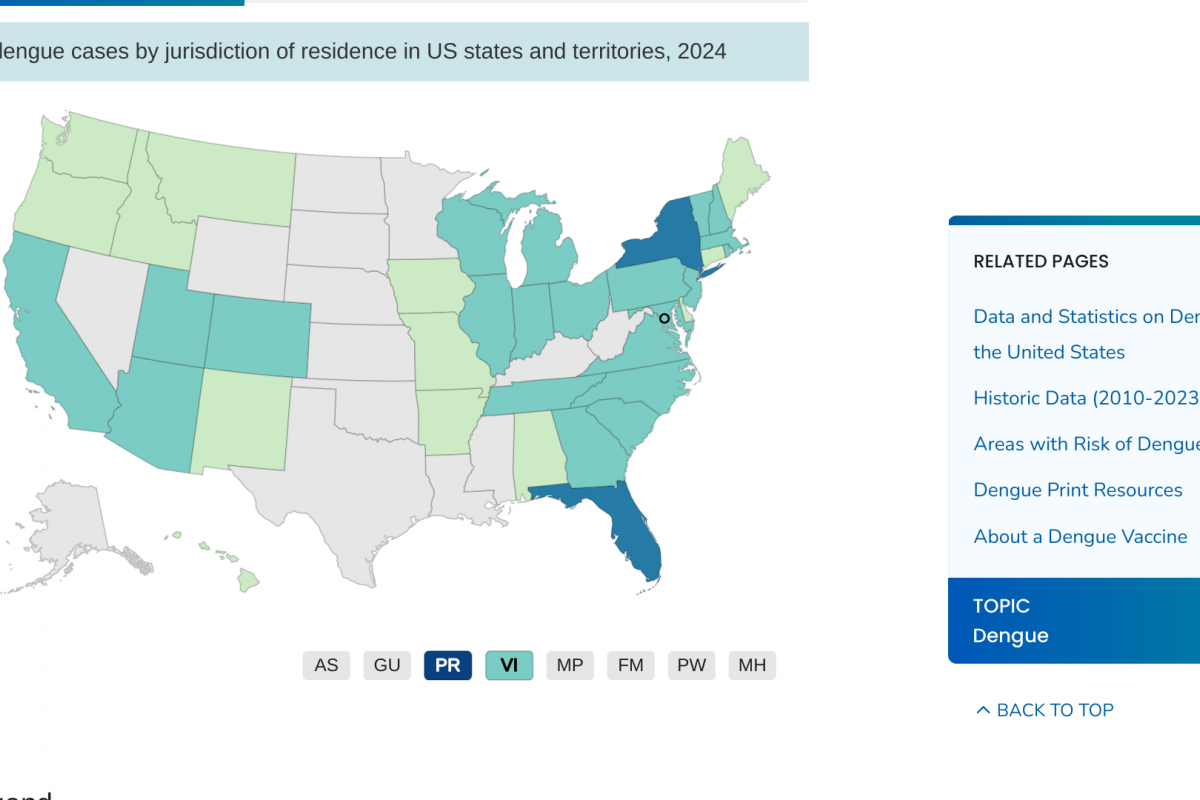
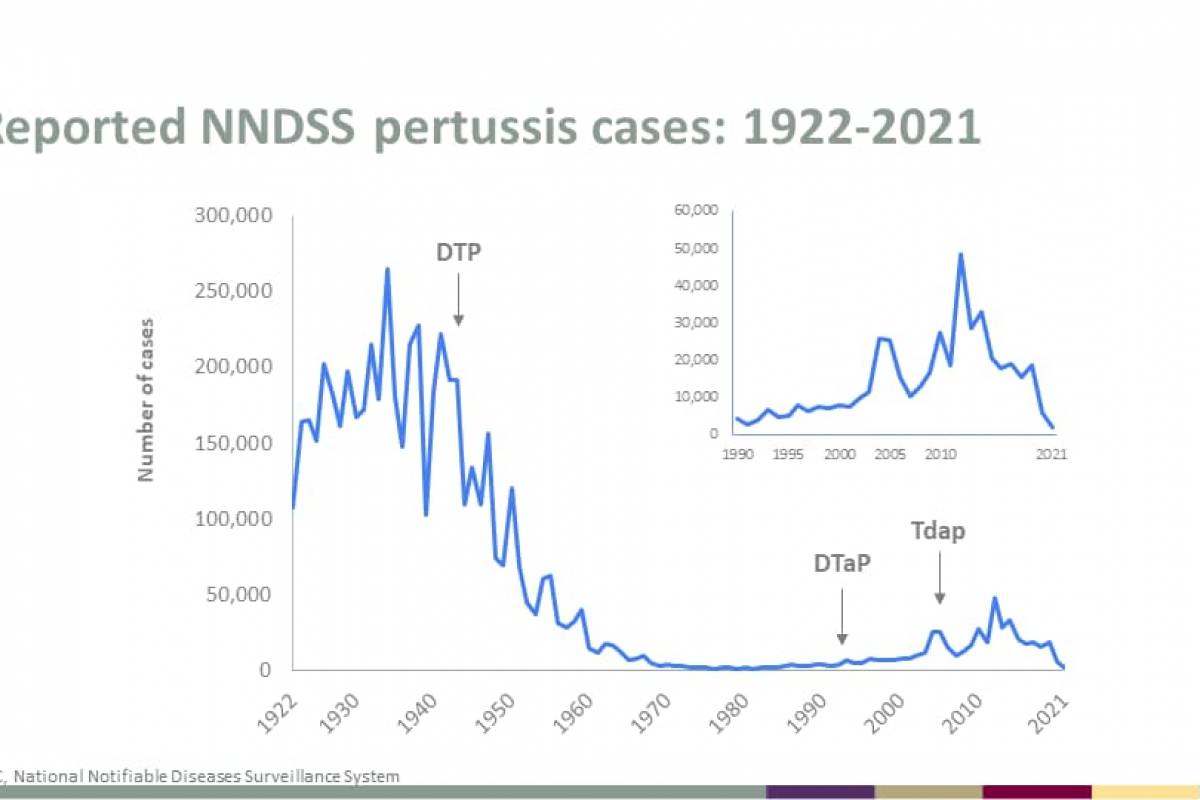
The U.S. Centers for Disease Control and Prevention (CDC) today announced an outbreak of chikungunya in Malé and Hulhumalé regions of Maldives.
Crisis24 recently reported 389 total cases from March to May 11, 2024.
On May 28, 2024, the CDC Level 2 Travel Health Advisory recommended that adults traveling to a destination with a current chikungunya outbreak be vaccinated.
The U.S. FDA recently approved Valneva SE's IXCHIQ® monovalent, single-dose, live-attenuated chikungunya vaccine.
Furthermore, the CDC says if you are pregnant, reconsider travel to Maldives, particularly if you are close to delivering your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
According to the CDC, newborns infected in this way or by a mosquito bite are at risk for severe illness, including poor long-term outcomes.
Additionally, people can protect themselves from chikungunya infection by preventing mosquito bites, including using insect repellent, wearing long-sleeved shirts and pants, staying in places with air conditioning, or using window and door screens.