A Cincinnati-based biopharmaceutical company developing transformational vaccines today announced the development of vaccine candidates targeting Mpox and Marburg virus disease (“MVD”).
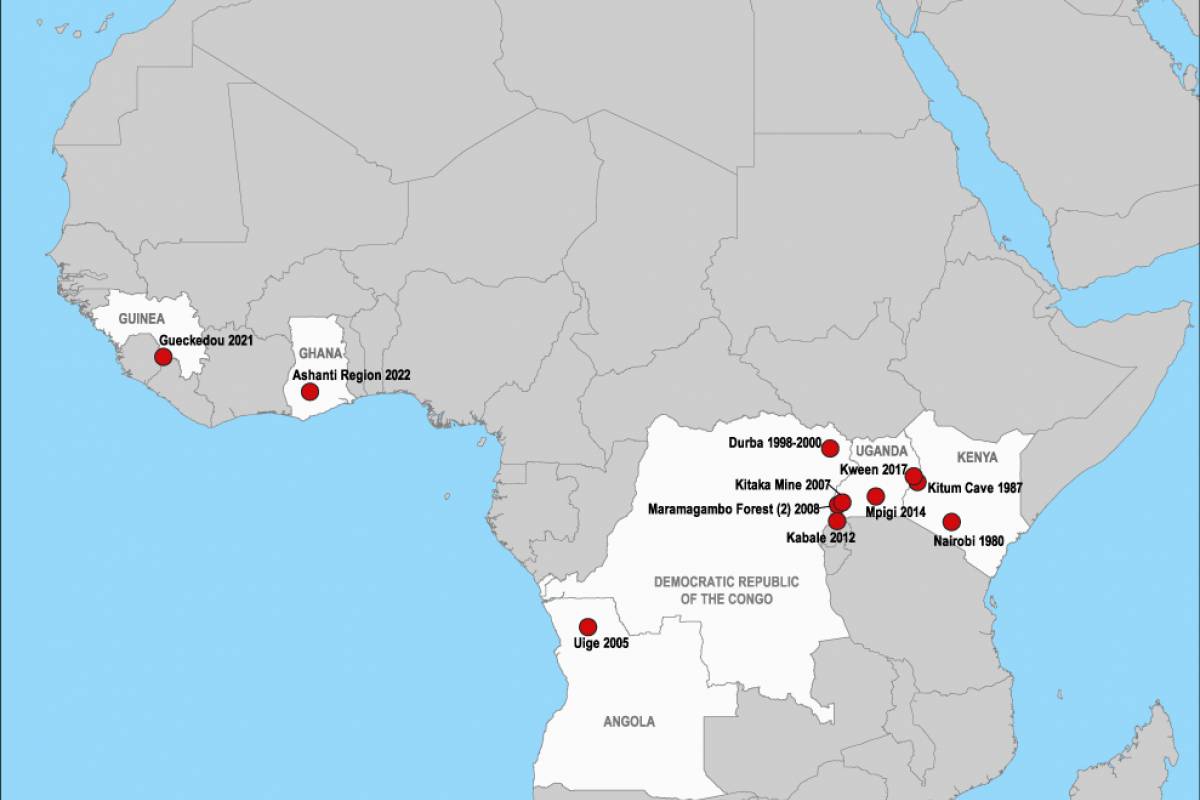
No vaccines or antiviral treatments are currently approved for MVD, which was first recognized in 1967.
As of 2023, Angola, DR Congo, Germany, Ghana, Guinea, Kenya, Serbia, South Africa, and Uganda have confirmed MVD cases.
Both candidate vaccines will utilize Blue Water Vaccines (BWV) Inc. norovirus shell and protrusion virus-like particle platform, which allows for the presentation of multiple antigens on the surface of either the S or P particle of a norovirus backbone.
In addition to monkeypox vaccine development, AbVacc will utilize its extensive expertise in MVD to develop a novel vaccine targeting the Marburg virus using BWV’s VLP platform.
“As various epidemics continue to emerge around the world, there has never been a better time to invest in the creation of preventative vaccines,” said Joseph Hernandez, Chairman and CEO of BWV, in a press release on February 1, 2023.
MVD is caused by either the Marburg or Ravn viruses, both from the same family as Ebola viruses and can cause outbreaks with high transmission and fatality rates.
According to the World Health Organization (“WHO”), Marburg spreads through human-to-human transmission via direct contact with the blood, secretions, organs, or other bodily fluids of infected individuals or contaminated surfaces.
Case fatality ratios of MVD can reach up to 88%, indicating a severe unmet need for preventative and therapeutic options, says the WHO.
Marburg vaccine candidates at listed on this webpage.