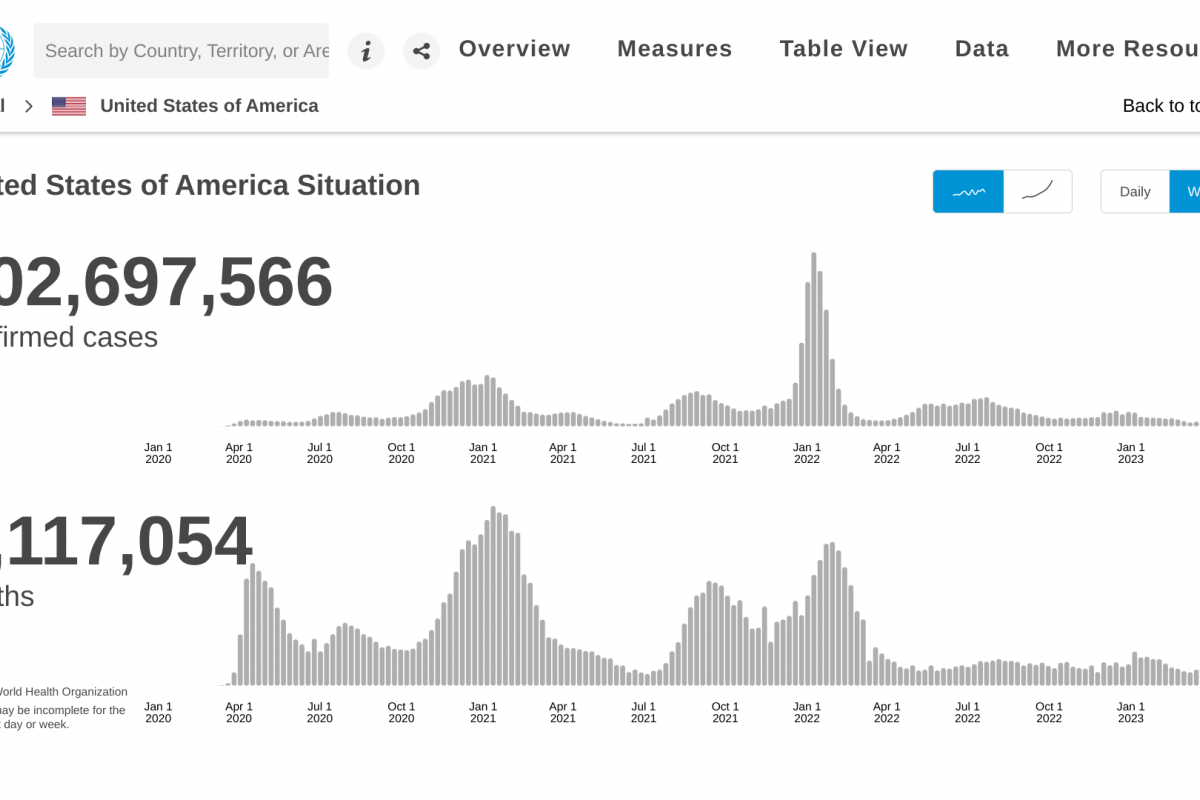
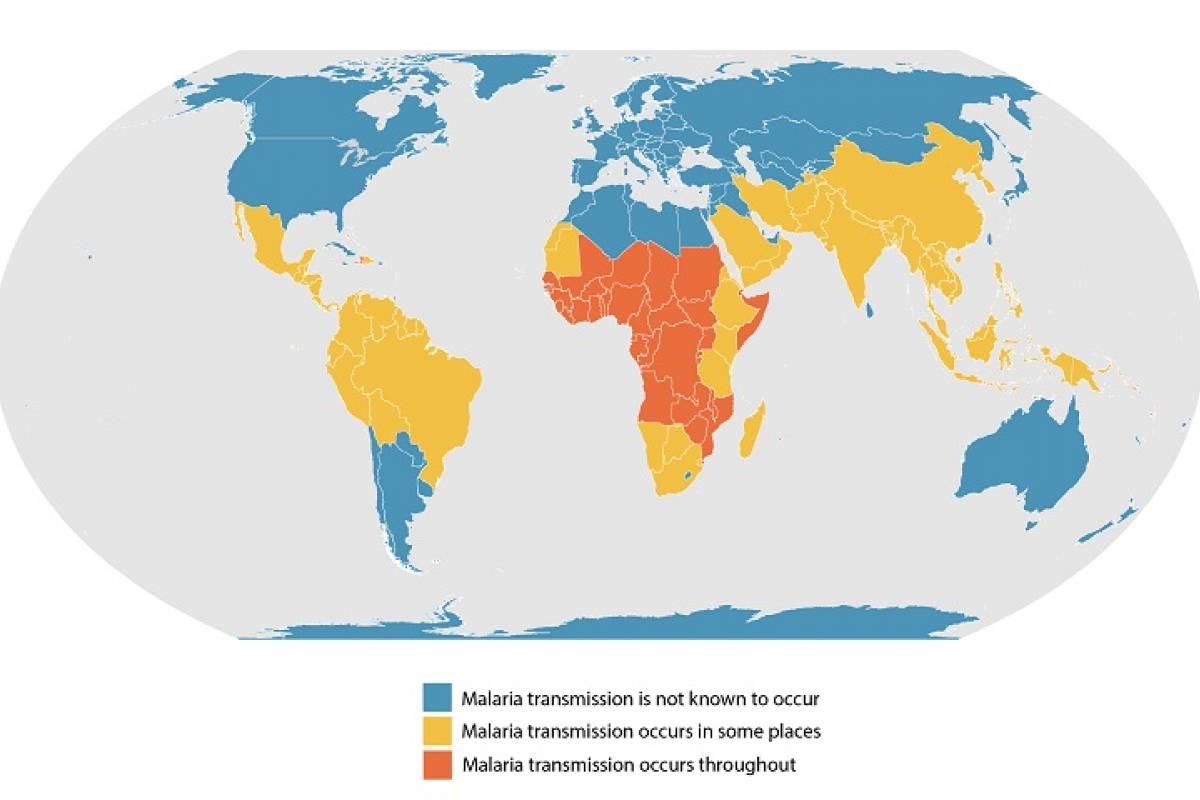
The New York State Department of Health (NYSDOH) recently confirmed the presence of poliovirus in Rockland County wastewater for the first time since October 2022.
This single positive result was collected in February 2023.
However, sequencing analysis by the U.S. Centers for Disease Control and Prevention (CDC) confirmed as of March 22, 2023, the presence of poliovirus in 101 positive wastewater samples, most linked to the individual case of paralytic polio among a Rockland County resident in 2022.
Transmission of this disease only continues if the overall polio vaccination rates remain low, says the CDC.
"It is our obligation to protect all our residents from debilitating and potentially fatal diseases," said Rockland County Executive Ed Day in a press release.
"I urge our residents to act now and protect yourselves, your family, and your community."
"Polio is preventable through the complete vaccination series."
As of mid-March, 19,282 doses of the polio vaccine have been administered to Rockland County residents.
In 2022, there were over 330,000 residents in Rockland County.
"While certainly, the number of doses is strong, the polio vaccine is a series of 4-doses, so it takes time to reach full protection," added Day.
"As I've said before, this is why it is crucial for schools and the New York State Education Department to maintain and enforce these required vaccinations."
"But families with unvaccinated children who are not yet of school age completely lack protection from this dreaded disease and must get on the schedule now."
Rockland County Department of Health (RCDOH) is actively working on strategies to increase vaccination rates, including working with the CDC and NYSDOH to perform daycare and school assessments and audits.
To enhance local detections, New York recently received $21.6 million in funding to expand its wastewater surveillance and infectious disease monitoring capabilities.
In 2023, this funding will increase the number of participating sewer sheds from 125 to over 215, reaching about 81% of the population of New York public sewer systems.
Moreover, with holiday travelers peaking this spring break, enhancing protection from polio is a priority, says the CDC.
For example, Israel's Ministry of Health also recently confirmed four children tested positive for poliovirus in Northern Israel.
With increased travel expected between New Yorkers and Israel over Passover 2023, the RCDOH reminds families there is a real risk of paralysis if they are unvaccinated.
Furthermore, adults who completed the polio vaccine series as children may receive a one-time polio booster dose before traveling abroad to any of the thirty countries reporting poliovirus detections.
And any New York resident who needs vaccination can obtain one by calling the RCDOH at 845-364-2520 or 845-364-2524.
Throughout the U.S., polio vaccines are offered by health clinics and community pharmacies as of March 28, 2023.