Search API
Marburg virus disease has been detected in the South Ethiopia Region, the first of its kind in the country, following laboratory testing of samples from a cluster of suspected cases of viral haemorrhagic fever.
As of November 14, 2025, the World Health Organization (WHO) has reported a total of 9 cases in the outbreak affecting Jinka town in the South Ethiopia Region.
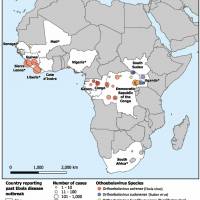
In the African region, previous Marburg outbreaks and sporadic cases have been reported in Angola, the Democratic Republic of the Congo, Ghana, Kenya, Equatorial Guinea, Rwanda, South Africa, Tanzania, and Uganda.
Marburg's initial outbreak was detected in Germany in 1967.
Marburg is a severe and often fatal illness transmitted to humans from fruit bats and spreads among people through direct contact with the bodily fluids of infected individuals or contaminated materials.
The WHO and national authorities are scaling up the response, including community-wide screening, case isolation, treatment, contact tracing, and public awareness campaigns to curb the spread of the Marburg virus, which belongs to the same family of viruses as Ebola virus disease.
Currently, no approved Marburg vaccines are available.
However, in April 2025, the Albert B. Sabin Vaccine Institute recently launched a multi-site Phase 2 clinical trial in the U.S. for its Marburg vaccine candidate, based on the cAd3 platform.
Currently, about five other Marburg vaccine candidates are being tested in clinical research.
Updated on November 17, 2025 - Organisations undertaking business in Ebola or Marburg-affected areas should register with the UK Health Security Agency returning workers scheme.

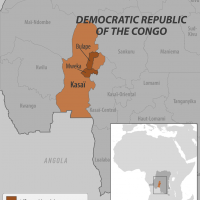
The World Health Organization (WHO today announced that in the Democratic Republic of the Congo (DRC), the first signs of the Ebola virus were detected in a maternity ward in Bulape, located in the Kasai Province.
A pregnant woman arrived showing unusual symptoms of this severe disease. The midwife on duty at the time assisted the woman through labour.
While handling the needle used on the patient, she accidentally pricked herself. A few days later, the midwife passed away, along with the mother and newborn.
Antho, the head of the maternity ward, was also present, commented in a WHO press release on November 14, 2025, "Not only was I wearing gloves, but each time I touched the patient, I went out and washed my hands with soap and water."
"It was hard to lose Juliette (midwife). We'd worked together for a long time, and even in the toughest moments, she supported the team. Her loss has left a big void."
The ongoing Ebola response in the DRC has focused on strengthening infection prevention along with other key outbreak control measures. This area has previously reported several Ebola outbreaks.
In public health emergencies, the WHO says infection prevention and control are pivotal in saving lives.
Currently, the DRC has access to Ebola vaccines and antibody treatments.

The World Health Organization (WHO) announced today that health authorities in Ethiopia are increasing their response and conducting further investigations following reports of suspected cases of viral hemorrhagic fever in the South Ethiopia Region.
As of November 13, 2025, the WHO has reported eight suspected cases in this region where about 7.5 million people live.
The South Ethiopia Regional State borders Kenya and South Sudan.
Laboratory testing is currently underway to determine the exact cause of these cases. Viral hemorrhagic fever is caused by several distinct families of viruses, including Marburg, Ebola, Crimean-Congo hemorrhagic fever, and Lassa fever.
Currently, only the Zaire Ebola virus has approved vaccines and antibody therapies.
In support of this investigation, the WHO has deployed an initial team of responders and delivered medical supplies to assist in the ongoing efforts to determine the cause of infection and halt further transmission.
Previously, the U.S. CDC included Ethiopia in its Travel Health Notices for malaria, measles, and polio issued in 2025.
The last Ebola Zaire patient in the Democratic Republic of the Congo (DRC) was recently discharged, marking a milestone in the efforts to end the this African country's 16th outbreak.
If no new cases are detected, this Ebola outbreak will be declared over in early December 2025.
In total, 64 cases (53 confirmed and 11 probable) have been reported since the outbreak was declared in Bulape health zone, in Kasai Province.
According to the World Health Organization (WHO), despite the challenges of distance, poor roads, and limited infrastructure, the Ministry of Health, with strong support from partners, acted swiftly to scale up outbreak response measures.
For example, more than 35,000 people have been vaccinated against Ebola in Bulape.
"The recovery of the last patient just six weeks after the outbreak was declared is a remarkable achievement that shows how strong partnership, national expertise, and determination have contributed to overcoming challenges to save and protect lives," said Dr. Mohamed Janabi, WHO Regional Director for Africa, in a press release on October 19, 2025.
In mid-September, the U.S. Centers for Disease Control and Prevention (CDC) issued Health Alert Network Health Advisory CDCHAN-00524 stating that the risk of Ebola spread to the United States is currently considered low.
The CDC says the ERVEBO® vaccine is approved for preventing the Ebola Zaire virus disease. However, it should only be given to people who meet specific criteria, such as visiting an outbreak zone.
Additionally, two approved treatments treat Ebola virus infection: Inmazeb™ and Ebanga™.
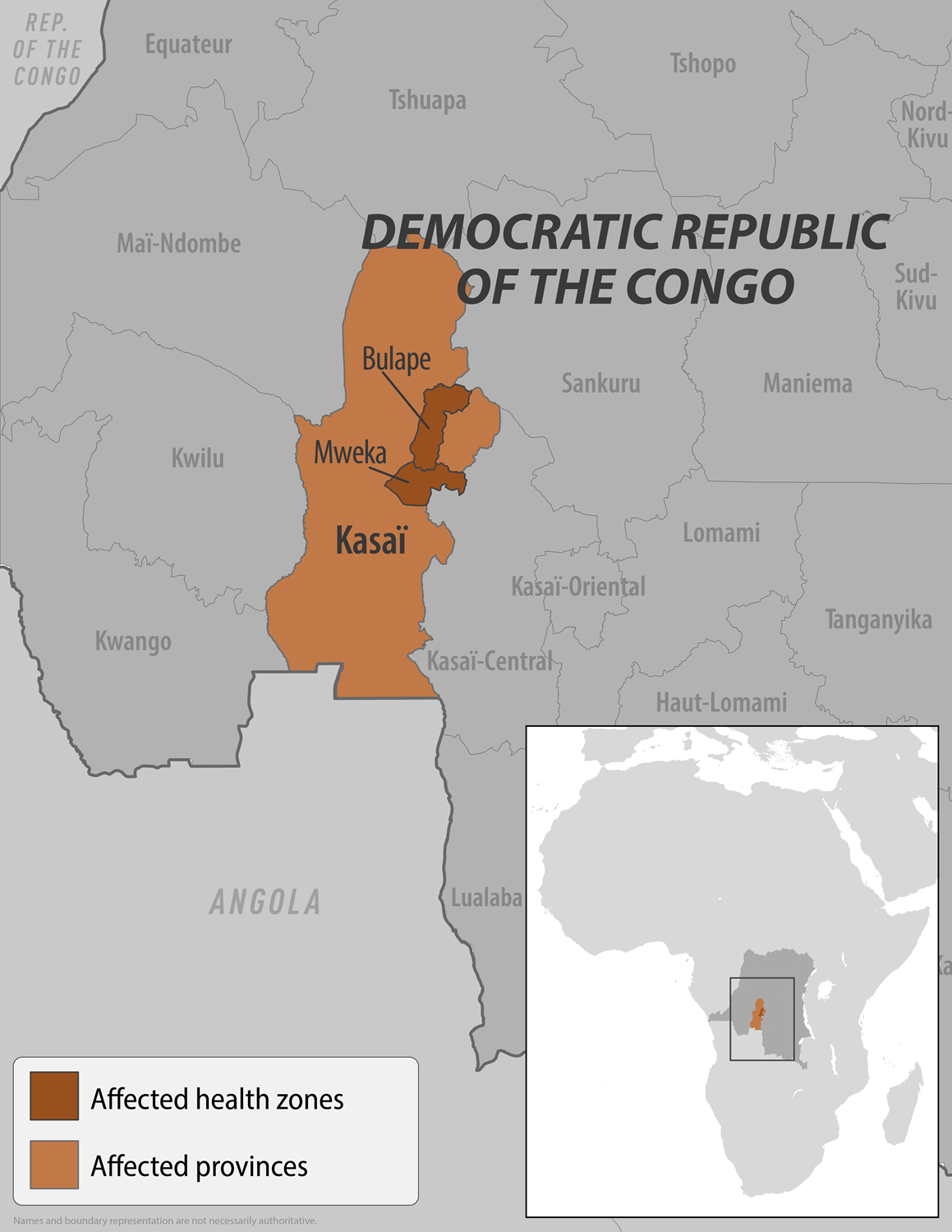
The 16th Ebola virus disease (EVD) outbreak in the Democratic Republic of the Congo (DRC) continues as of October 2025; however, there are signs of a notable decline in transmission.
As of the end of September 2025, a total of 64 cases (53 confirmed and 11 probable), including 42 deaths (31 confirmed, 11 probable), have been reported from Bulape Health Zone, Kasai Province, Democratic Republic of the Congo.
Since the World Health Organization (WHO) Situation Report #2, a total of seven new EVD cases have been reported. The latest cases were detected across three areas within Bulape Health Zone, namely, Bulape, Mpianga, and Dikolo.
During the same reporting period, seven deaths occurred among newly identified and previously hospitalized cases.
The overall case fatality ratio (CFR) during this, the 16th EVD outbreak in the DRC, is 65.6% (64 cases, 42 deaths).
The U.S. CDC recently stated that, although not commercially available in the USA, an FDA-approved vaccine exists for the prevention of Ebola virus (species Orthoebolavirus zairense). It is currently available to select individuals in specific occupations (e.g., outbreak responders).
Contact your healthcare provider to discuss eligibility and access to the Ebola vaccine (Ervebo), as recommended by the CDC.
Additionally, the CDC recommends vaccinations for measles, polio, and several routine diseases before visiting the DRC in 2025.
The U.S. Centers for Disease Control and Prevention (CDC) today issued Health Alert Network Health Advisory CDCHAN-00524 focused on the 16th outbreak of Ebola virus disease (EVD) in the Democratic Republic of the Congo (DRC).
Currently, no suspected, probable, or confirmed EVD cases related to this EVD outbreak have been reported in the United States or outside of the Bulape and Mweka health zones within the Kasai Province, DRC.
The CDC stated on September 18, 2025, that the risk of spread to the United States is currently considered low.
As a precaution, this Health Advisory summarizes CDC recommendations for U.S. public health departments, clinical laboratories, and healthcare workers about potential EVD case identification, testing, and biosafety considerations in clinical laboratories.
An Ebola vaccine (ERVEBO®) is approved for preventing Zaire Ebola virus disease; it should only be given to patients who meet specific criteria.
Two approved treatments are currently available to treat Ebola virus infection: Inmazeb™ and Ebanga™.
To alert international travelers, on September 8, 2025, the CDC issued a Level 1, Travel Health Notice, for people traveling to the DRC.
The CDC recommends that all travelers to the affected health zones in DRC avoid contact with ill people during travel and monitor themselves for symptoms of EVD while in the outbreak area and for 21 days after leaving.
Travelers who develop symptoms during this time should self-isolate and contact their local health authorities or a healthcare provider.
At this time, the CDC is not recommending additional assessments or monitoring of travelers arriving from the DRC by jurisdictional health departments, unless specified in the existing guidance provided.
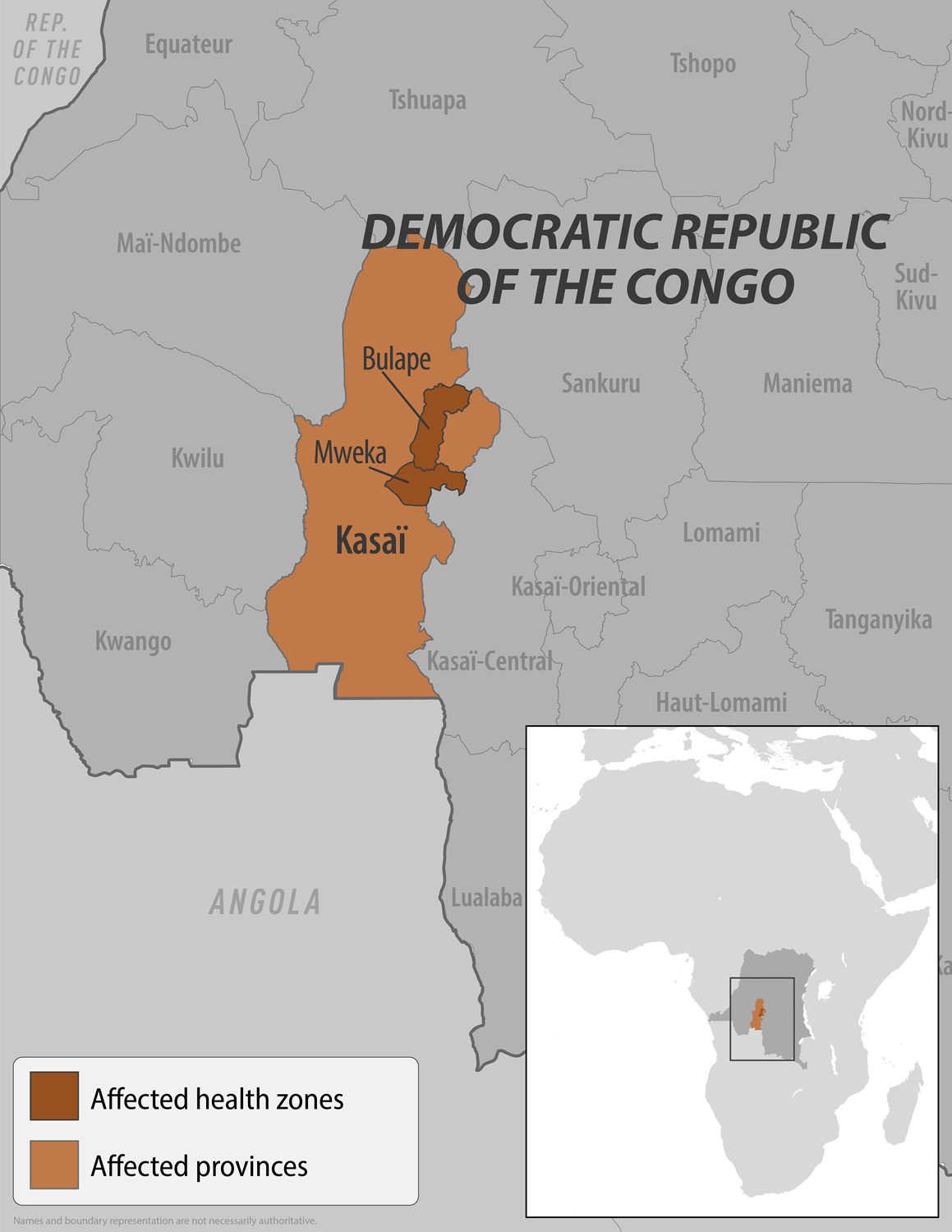
Vaccination of frontline health workers and contacts of people infected with Ebola virus disease has begun in Bulape health zone in the Democratic Republic of the Congo (DRC) 's Kasai Province, where an outbreak of the disease was declared.
The World Health Organization (WHO) stated on September 14, 2025, that an initial 400 doses of the Ervebo® Ebola vaccine, prepositioned in the capital, Kinshasa, have been delivered to Bulape, one of the current hotspots of the DRC's 16th Zaire Ebolavirus outbreak.
Additional Ervebo doses will be delivered to the affected localities in the coming days.
In addition, treatment courses of the Ebanga™ monoclonal antibody therapy (Mab114) drug have also been sent to treatment centres in Bulape for clinical care.
In the outbreak area, WHO has so far deployed 48 experts in disease surveillance, clinical care, infection prevention and control, logistics, and community engagement.
In countries neighboring the DRC, the WHO is working with national authorities to bolster operational readiness, enabling the rapid detection of Ebola cases and the prompt initiation of measures to curb further spread of the lethal virus.
As of September 15, 2025, the WHO assesses the overall public health risk posed by the ongoing outbreak as high at the national level, moderate at the regional level, and low at the global level.
To alert international travelers, the U.S. CDC issued a Level 1 - Practice Usual Precautions, Travel Health Advisory regarding this Ebola outbreak.
The CDC wrote on September 8, 2025: "While not commercially available, there is an FDA-approved vaccine for the prevention of the Zaire Ebola virus." It is presently available to select individuals in specific occupations (e.g., outbreak responders).
For more information on vaccines, please consult with your healthcare provider to discuss eligibility and access to the Ebola vaccine.
- 1 of 11
- next ›