Search API
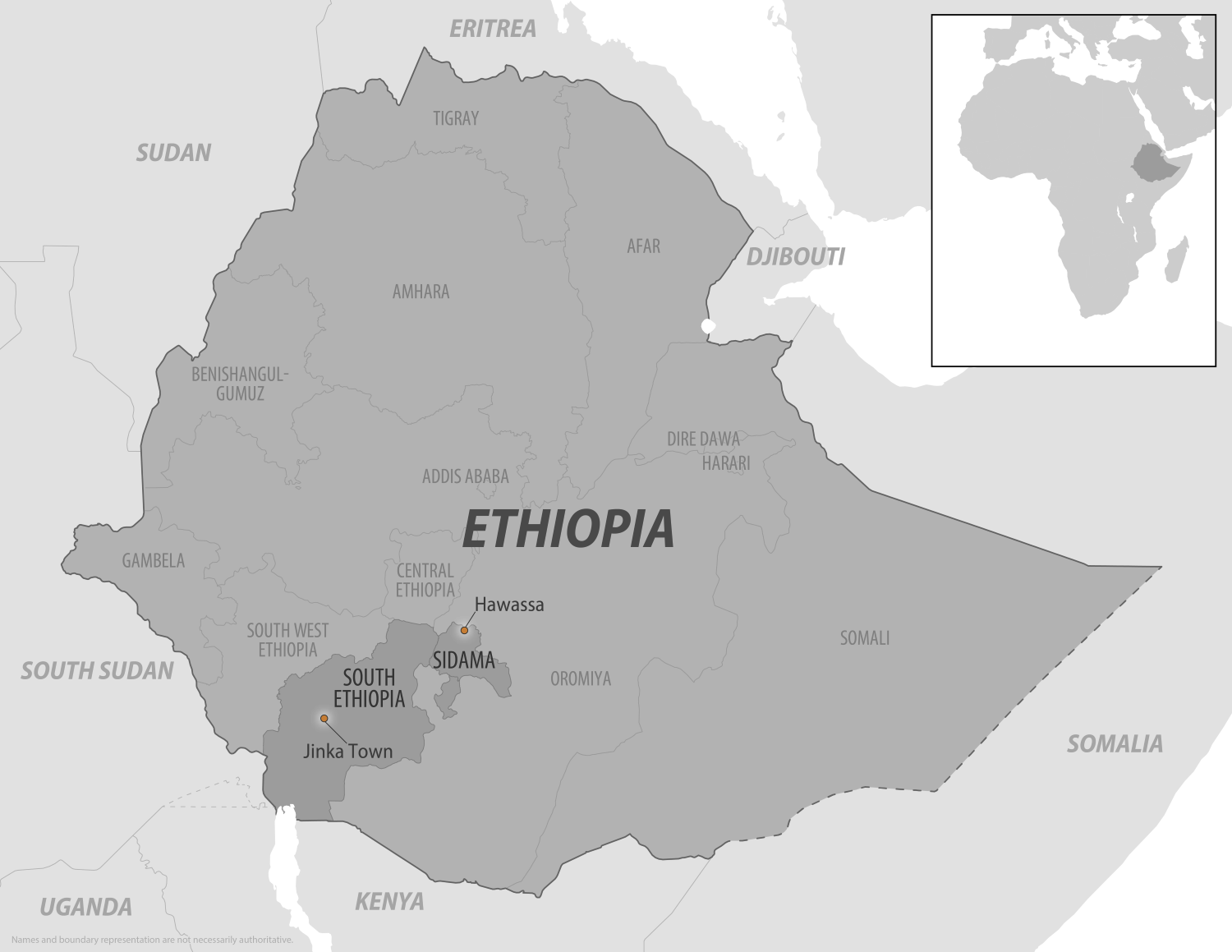
In late 2025, Ethiopia experienced its first outbreak of Marburg virus disease (MVD) in its southern regions.
As of December 12, 2025, Ethiopia's Ministry of Health posted on Facebook that there have been 14 confirmed cases of MVD with nine related fatalities.
Accordingly, the UK FCDO advises against all but essential travel within 5km of the towns of Jinka and Hawassa due to the ongoing MVD outbreak.
In early December, the U.S. CDC updated its Level 1 travel advisory, stating to watch for symptoms of Marburg while in the outbreak area and for 21 days after leaving Ethiopia. If you develop fever, chills, headache, muscle aches, rash, chest pain, sore throat, nausea, vomiting, diarrhea, or unexplained bleeding or bruising, contact a healthcare provider.
Since its initial outbreak in 1967, no suspected, probable, or confirmed MVD-related cases have been reported in the United States or the United Kingdom. Previous MVD outbreaks have been confirmed in Europe and other African countries.
Currently, no preventive vaccine has been approved for human use. However, Marburg vaccine candidates are conducting clinical research as of December 15, 2025.
The Pan American Health Organization (PAHO) recently released a briefing note informing countries about the increasing circulation of the influenza A(H3N2) subclade K (J.2.4.1) virus.
The PAHO noted in a media release that the genetic evolution observed in subclade K is part of the natural variation in seasonal influenza viruses.
As of December 12, 2025, in North America, particularly in the United States and Canada, there has been a gradual increase in detections of subclade K. As of now, similar circulation levels have not been reported in South American countries.
While evidence on vaccine effectiveness for the current season remains limited, preliminary data from Europe suggest that vaccination continues to offer comparable protection against severe disease, including hospitalization, wrote the PAHO.
The ECDC previously wrote that compared to previous years, influenza is increasing unusually early in the EU/EEA, with A(H3N2) driving the increases in recent weeks. Even if a less well-matched A(H3N2) virus dominates this winter, the vaccine is still expected to protect against severe disease.
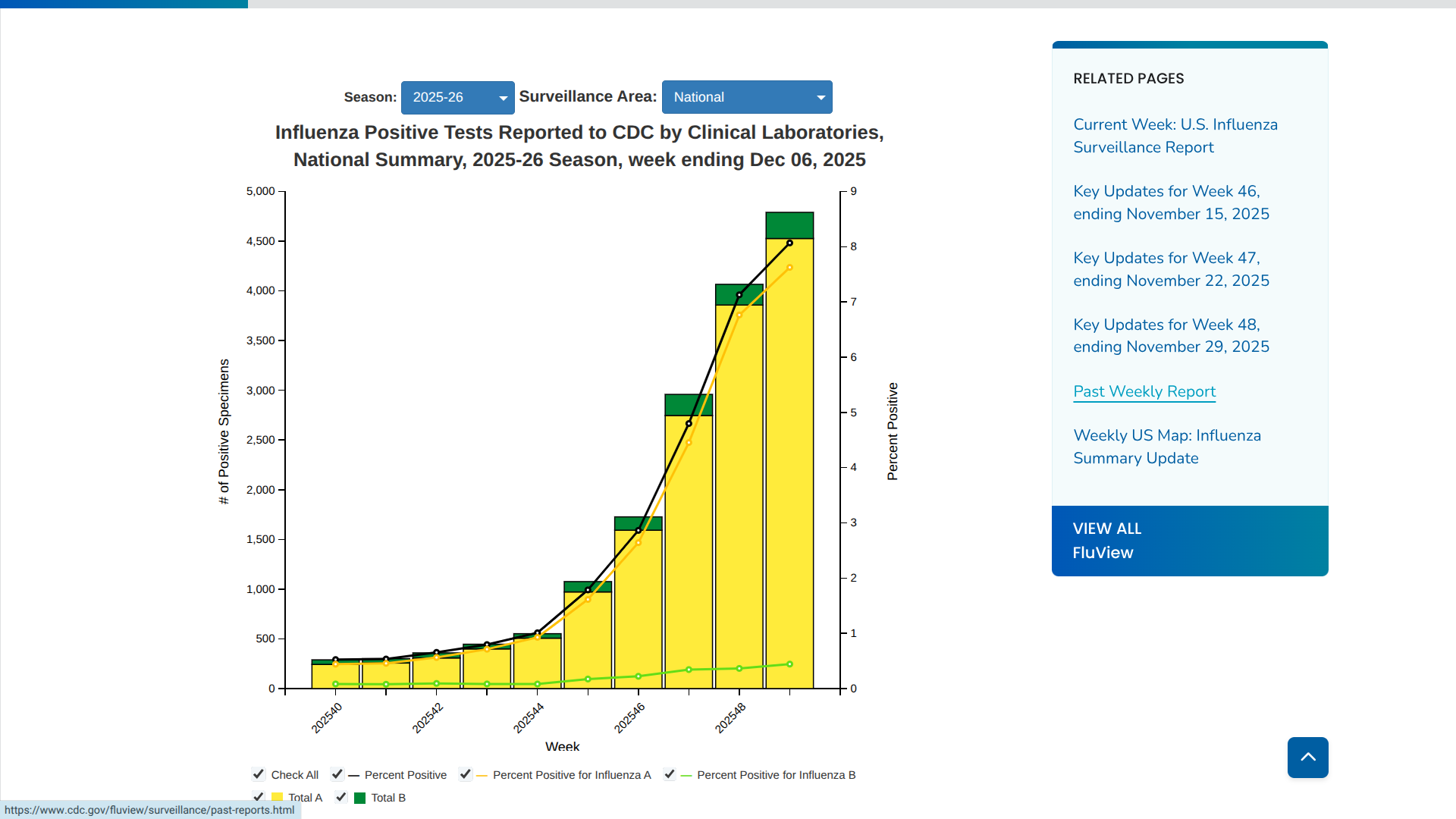
The U.S. CDC wrote last week that subclade J.2.4.1, renamed "H3N2 subclade K," was identified in August 2025. Of the 401 influenza A viruses subtyped during Week #49 in the USA, 14.% were influenza A(H1N1)pdm09, and 86.0% were A(H3N2).
These viruses have slight changes in their hemagglutinin gene and have been antigenically characterized as "antigenically drifted" in comparison to the virus selected as the A(H3N2) component of the U.S. 2025-26 seasonal influenza vaccines.
The CDC reaffirmed its recommendation that most people in the USA, and those traveling abroad for the winter holidays, get an annual flu shot.
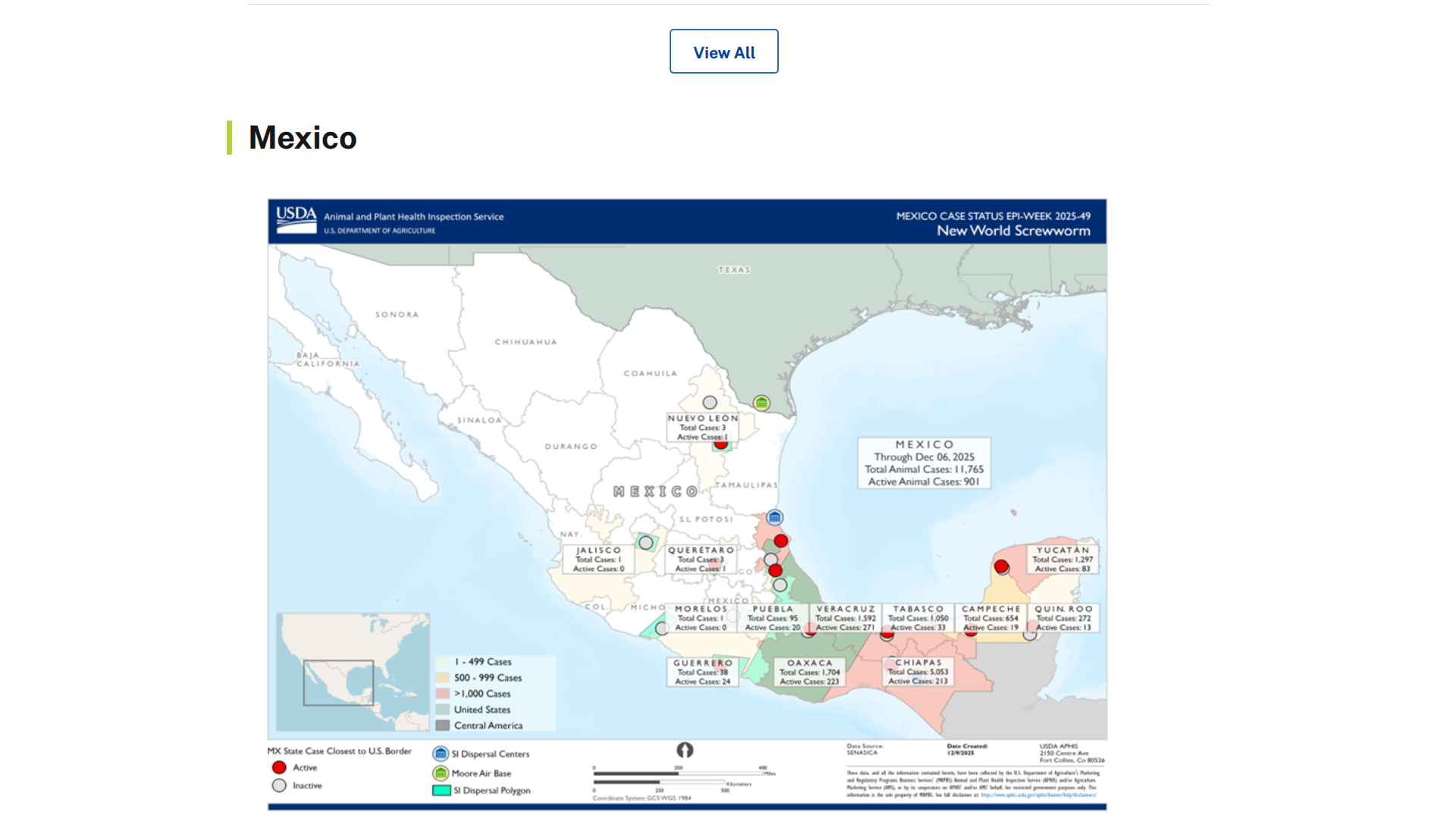
Local media in Mexico have reported human cases of myiasis associated with the northern expansion of New World Screwworms (NWS).
As of early December 2025, reports of myiasis cases in Chiapas, Oaxaca, Yucatán, Campeche, and Tabasco, totaling around 92 human cases, including five related fatalities.
Throughout the Region of the America, 1,000 NWS cases in people have been confirmed in 2025.
According to the United States government, NWS has not crossed into the state of Texas and is not currently present in the USA. However, there has been one confirmed case of NWS in a person who returned to the USA after traveling to El Salvador.
The U.S. CDC says Infestations by NWS maggots can cause painful and foul-smelling wounds. If not caught early and treated, it can lead to extensive tissue damage and potentially death.
The CDC recommends that healthcare providers, if they suspect a patient has an NWS infestation, report it immediately to their local or state health department.
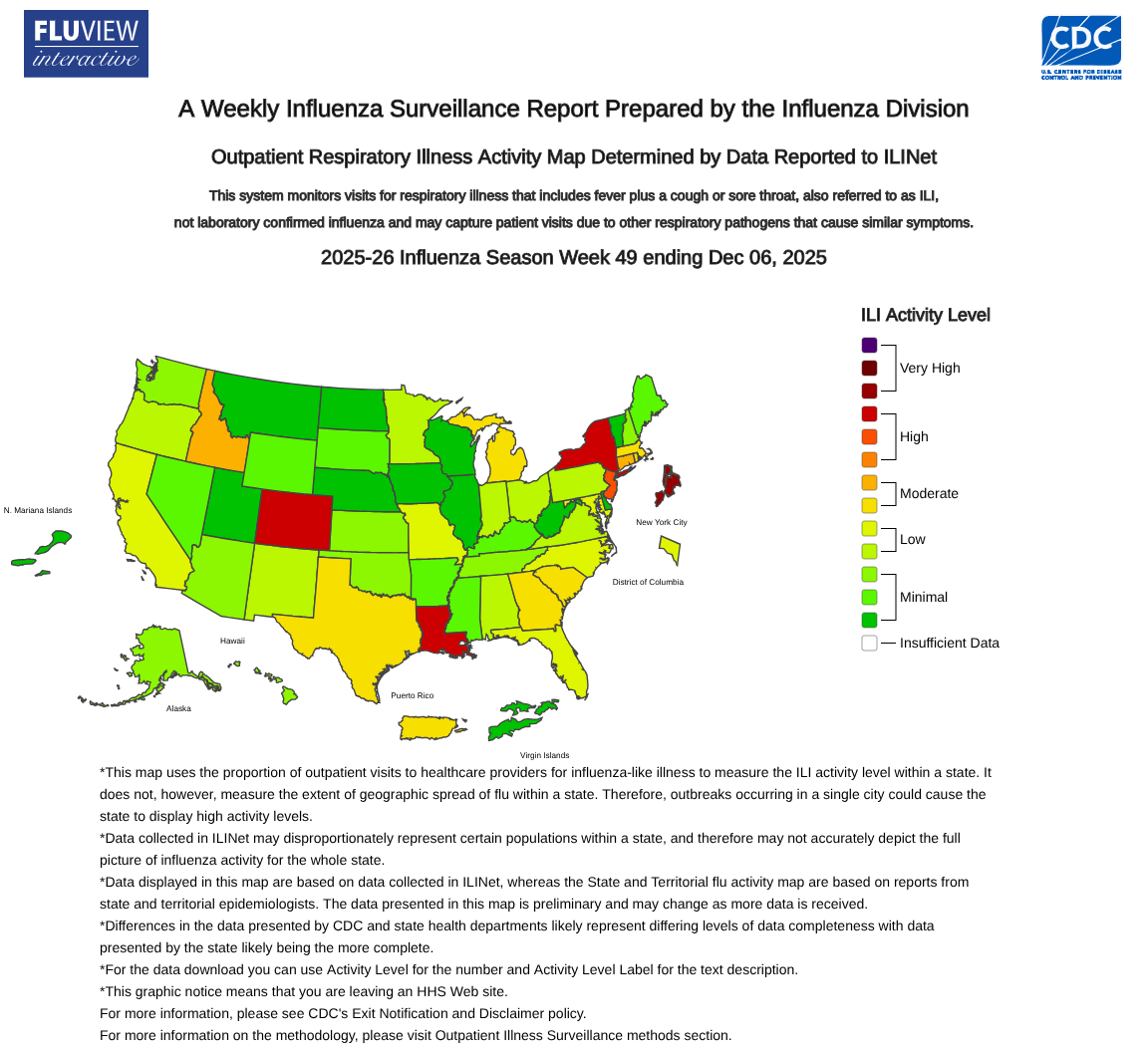
As the first part of the 2025-2026 influenza season comes to a close, three states have been classified as having flu outbreaks by the U.S. government.
According to the U.S. CDC FluView Week 49 report, ending December 6, 2025. Colorado, Louisiana, and New York are seeing significant numbers of influenza detections.
Additionally, the CDC reported two influenza-associated pediatric deaths during Week 49.
Furthermore, there is an early indication that this season's flu shots are effective.
Early estimates of 2025-26 influenza vaccine effectiveness in England against influenza-associated hospitalization remained within expected ranges of 70-75% for children and 30-40% for adults, suggesting that influenza vaccination remains an effective tool in preventing influenza-related hospitalizations this season.
The CDC recommends that everyone 6 months and older who has not yet been vaccinated this season get an annual influenza vaccine. This advice is especially valid when living in or visiting an area with a flu outbreak.
As of December 14, 2025, about 127 million doses of influenza vaccine have been distributed in the USA this season, and they are readily available at travel clinics and pharmacies.
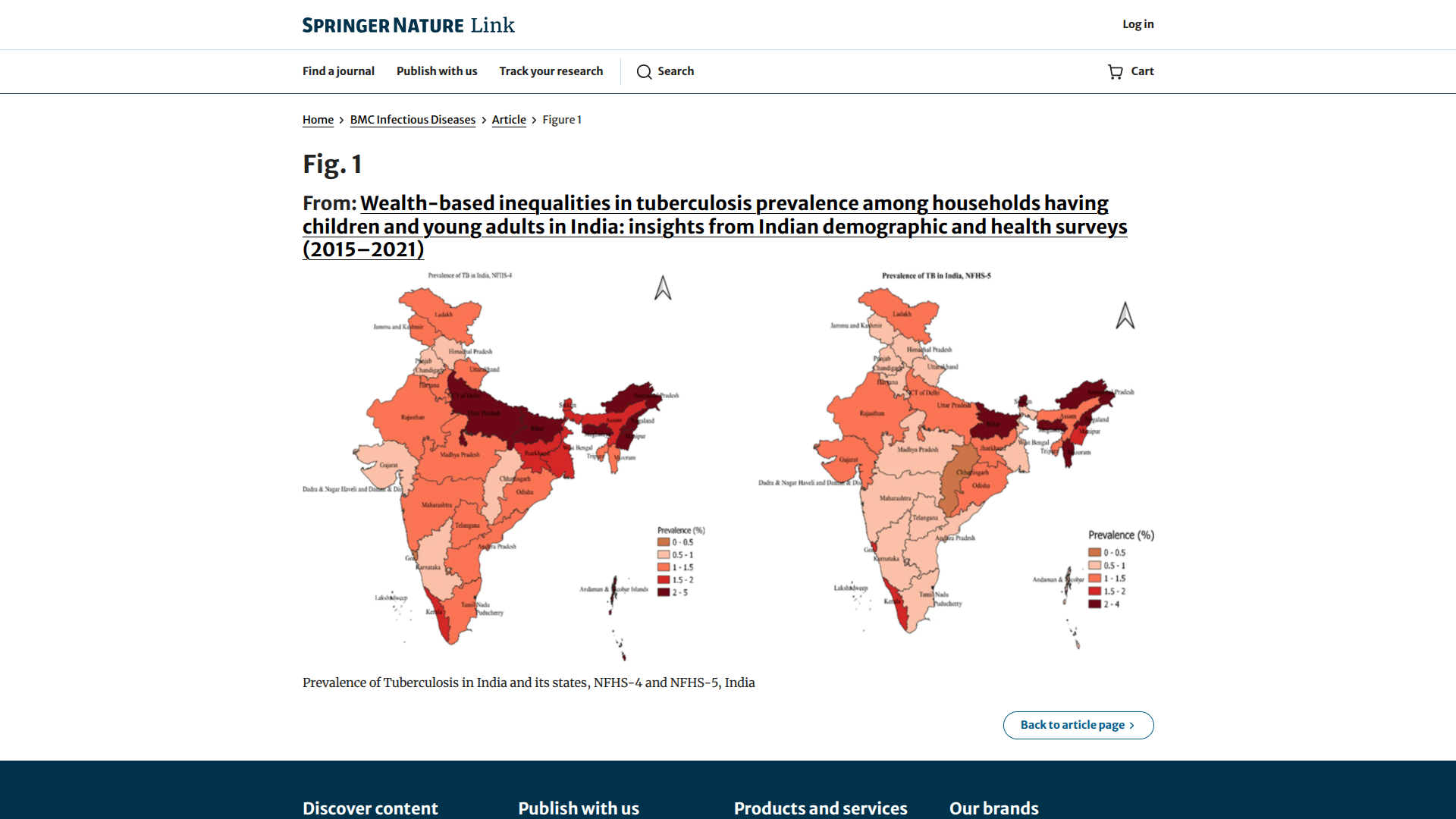
India's tuberculosis (TB) incidence has recently been reduced by 21%, almost double the pace observed globally, as per the World Health Organization's Global TB Report 2025.
Similarly, India's TB mortality rate has decreased, reflecting a near-tenfold increase in government funding to the TB program, which has focused on high-risk areas such as Uttar Pradesh, Maharashtra, Bihar, Madhya Pradesh, and Delhi.
Since its launch in December 2024, India's flagship TB elimination mission, the TB Mukt Bharat Abhiyan, has achieved extensive reach, screening over 19 crore vulnerable individuals across the country, leading to the detection of over 24.5 lakh TB patients, including 8.61 lakh asymptomatic TB cases.
Since TB is a vaccine-preventable disease, India offers access to the 100-year-old Bacille Calmette-Guérin vaccine.
These proactive approaches draw on both global and local evidence, underscoring India's commitment to science-based solutions to reduce the severity of this disease.
Unfortunately, the provisional data from the U.S. CDC for 2024 showed 8% increase from 2023, reaching the highest count since 2011, with rates rising across most age groups.
However, the CDC's week #49 data indicates a position trend in 2025, with only 8,489 TB cases confirmed as of December 6, 2025.
While access to the BCG vaccine in the U.S. is limited, recent shortages have been resolved with recombinant versions like rBCG.
As of December 13, 2025, the CDC doesn't recommend it for general use because of false-positive TB skin test results. However, healthcare providers can access the vaccine through special programs when visiting TB-outbreak areas in India.
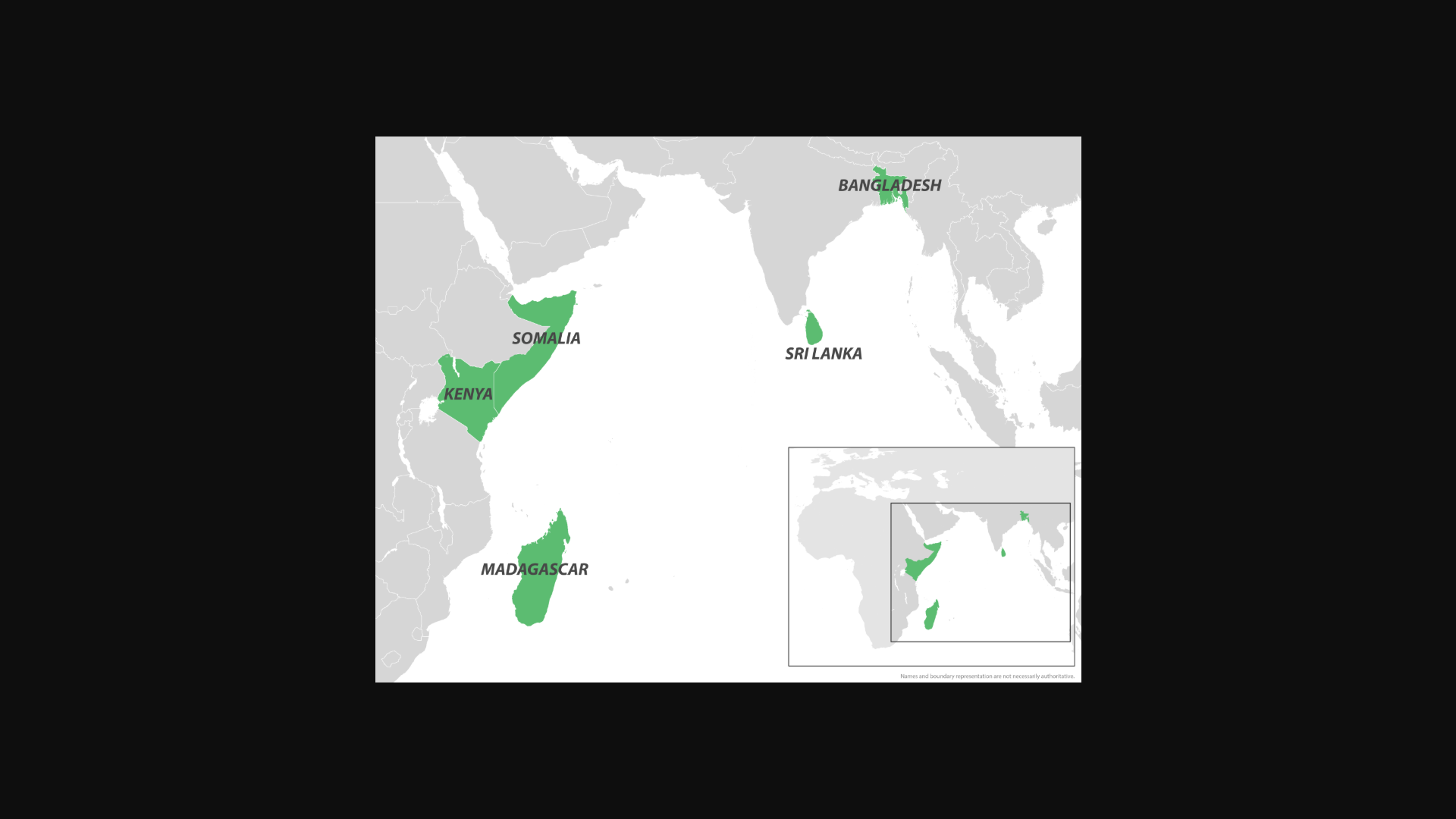
Throughout 2025, Chikungunya fever outbreaks have been reported in countries that border the Indian Ocean. From Madagascar to France's La Réunion Department, locally-acquired and travel-related cases have set new records.
To alert international travelers, the U.S. Centers for Disease Control and Prevention (CDC) recently issued a Level 2 - Practice Enhanced Precautions, Travel Health Notice regarding the Democratic Socialist Republic of Sri Lanka's ongoing Chikungunya fever outbreak.
Sri Lanka is located just south of India, home to about 21 million residents, and welcomed over 2 million visitors in 2024.
Earlier in 2025, a non-peer-reviewed study identified virus mutations that may be the source of these outbreaks.
These researchers wrote in May 2025 that the ongoing large outbreak in Sri Lanka is due to the Indian Ocean lineage and the E1:K211E/E2:V264A sublineage of the Chikungunya virus, which has acquired specific, previously uncharacterized mutations.
As of December 8, 2025, the CDC says that if you are pregnant, you should reconsider travel to the affected areas, particularly if you are close to delivering your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
Furthermore, newborns infected in this way or by a mosquito bite are at risk for severe illness, including poor long-term outcomes.
As of December 13, 2025, the CDC recommends vaccination with a U.S. FDA-approved vaccine for travelers visiting an area with a Chikungunya outbreak. Vaccines are commercially available at travel clinics in the USA.
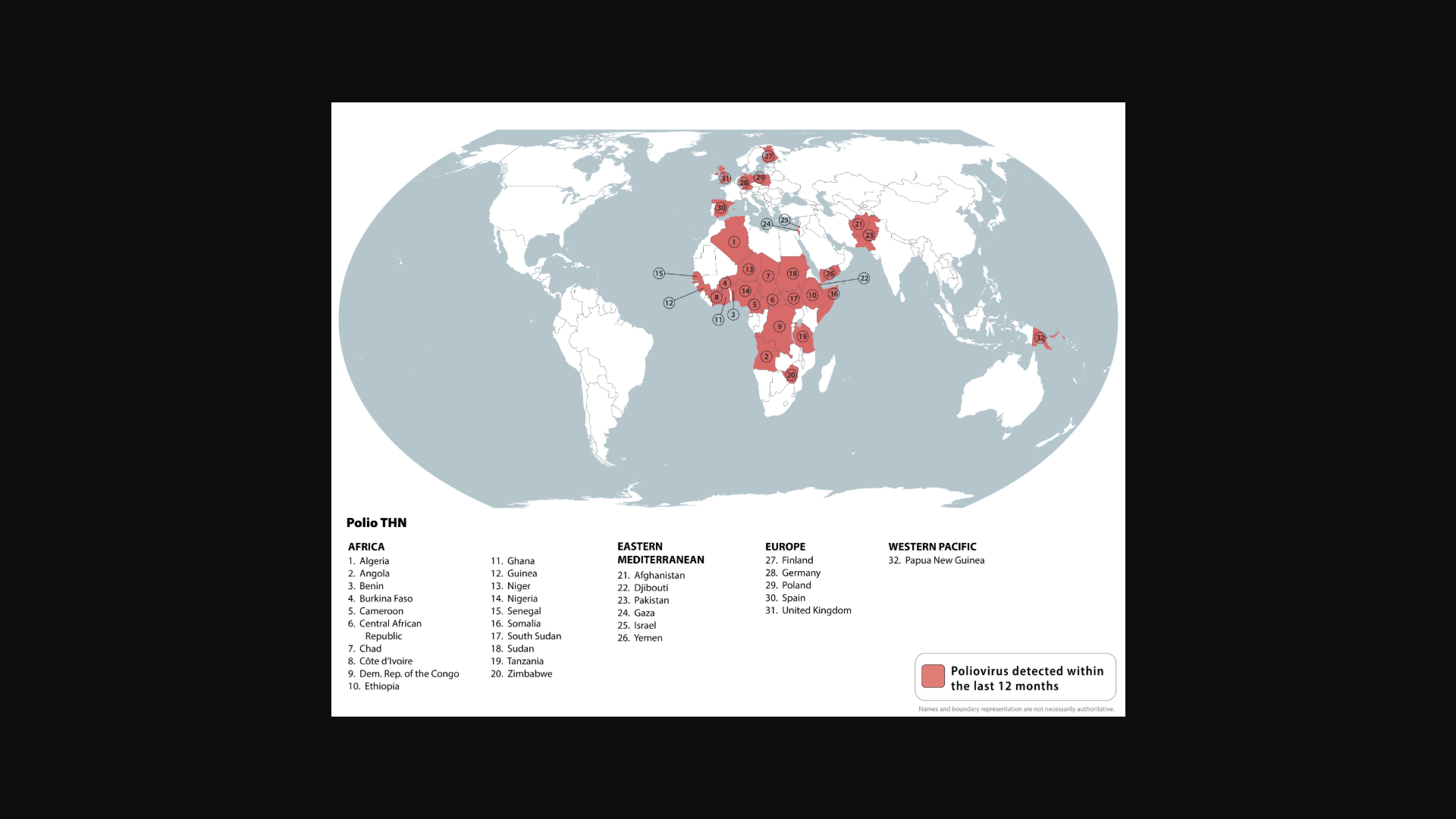
International leaders, philanthropists, and global health partners announced today a collective $1.9 billion to advance polio eradication. This funding is essential as wild poliovirus is now endemic in only two countries, but outbreaks of variant poliovirus still threaten children in over 30 countries.
According to a press release from the Global Polio Eradication Initiative (GPEI) on December 8, 2025, the funds will accelerate efforts to reach 370 million children annually with polio vaccines and strengthen health systems in affected countries to protect children from other preventable diseases.
This includes approximately $1.2 billion in newly pledged funds, reducing the remaining resource gap for the GPEI 2022-2029 Strategy to $440 million.
The pledges announced today reaffirm international resolve to finish the job and protect future generations from a disease that once paralyzed 1,000 children every day across 125 countries.
"The fight to end polio shows what is possible when the world invests together in a shared goal. We're 99.9 percent of the way there – but the last stretch demands the same determination that got us this far," said Bill Gates, Chair of the Gates Foundation. "This renewed funding will help us cross the finish line and strengthen the systems that protect children from this terrible disease for good."
Success would make polio just the second human disease ever eradicated—after smallpox—and is projected to save the world more than $33 billion by 2100 compared to the ongoing cost of outbreak control.
The Strategic Advisory Group of Experts on immunization (SAGE) recently endorsed two critical innovations for polio eradication.
SAGE recommended that fractional doses of Sabin-based inactivated polio vaccine (IPV) be used in the same way as fractional doses of Salk-based IPV – helping stretch supply and reach more children.
SAGE also backed the broader rollout of novel oral polio vaccine type 2 (nOPV2) to help stop persistent outbreaks of circulating variant poliovirus type 2 in some of the toughest places.
In 2024, SAGE recommended that, where feasible, the use of both IPV and nOPV2 vaccines be employed for initial outbreak-response vaccination campaigns.
In the United States, the IPV is offered at travel clinics, and booster shots are suggested for certain travelers.
- 1 of 405
- next ›