Search API
A research study was recently conducted to assess the safety and tolerability of the tetravalent live-attenuated dengue vaccine, which is commercially known as Qdenga®. The study provides important insights into reactogenicity and may help improve vaccination strategies in dengue-naïve populations.
The study results were published in the Journal of Travel Medicine on February 2, 2025. Vaccine-related reactions were frequently reported, predominantly after the first dose in dengue-naïve participants.
While vaccine coadministration was a common strategy, it did not significantly increase side effects.
After the first dose, 51% of the participants reported systemic reactions, such as headache (40% (190/474)), weakness (40% (189/474)), and malaise (32% (154/474)), which were most pronounced between days 7 and 11 after vaccination.
After the second dose, localized signs and symptoms such as pain at the injection site (22% (n = 55/250)) were more common. Fever was more common after the first dose (20% (96/474)) vs. 2% (6/250) after the second.
A total of 334 (28%) coadministrations were reported, whereby AEs were reported in 47% (157/333) of participants, with the highest prevalence observed when combined with the Japanese encephalitis vaccine (56.8%, (42/74)).
Differences in age groups were observed, with decreased reactions in older people (≥ 65 years).
As of February 2025, Qdenga is not offered in the United States and is in limited supply globally.
Later this year, Butantan Institute's single-dose, tetravalent, live attenuated Butantan-DV dengue vaccine may become available in Brazil, where it conducts phase 3 clinical trials.
The Victoria Department of Health (VDH) recently confirmed that a human case of Japanese Encephalitis Virus (JEV) had been identified in a resident of northern Victoria.
This is the first case in Victoria in 2025.
On January 20, 2025, Dr. Christian McGrath, VDH's Acting Chief Health Officer, issued an alert stating that residents and visitors to northern Victoria, mainly inland riverine regions and near the Murray River, are potentially at higher risk of infection and should take measures to prevent mosquito bites.
Victoria is Australia's second-most-populated state, with a population of over 6.9 million. In 2024, it welcomed about 12 million visitors, many visiting the city of Melbourne.
Since January 2021, 45 people in Australia have been infected with JEV outbreaks, resulting in seven deaths.
In June 2023, the Joint National Japanese Encephalitis Virus Outbreak Response Plan became effective.
"Historically, the risk of contracting JE in Australia has been limited to the Torres Strait region near Papua New Guinea. However, since 2021, new cases have been confirmed in southeastern Australian states, including Victoria, where infections have occurred in rural areas surrounding the Murray River," Jeri Beales, MSN, RN, informed Vax-Before-Travel News.
"If your vacation itinerary takes you to popular tourist areas in major Australian cities, you most likely do not need the JE vaccine."
"But if you plan to stay in risk areas for weeks to months, then vaccination is recommended," added Beales, MSN, RN, leads Destination Health Clinic, a Boston-area travel health provider specializing in health education and vaccination for international travelers.
Since JEV is a vaccine-preventable disease, Valneva SE's JESPECT® vaccine is offered free of charge in Australia for specific groups at higher risk of exposure to the virus. However, due to the significant global demand for the vaccine, access is restricted to those most at risk.
In February 2025, IXIARO® will be the only JEV vaccine commercially offered at travel clinics and pharmacies in the United States. The vaccine requires two doses for U.S. travelers, usually given four weeks apart.
To protect its military staff from this mosquito-transmitted disease, the U.S. Department of Defense recently ordered $32.8 million worth of IXIARO.
Note: This VBT News article was updated on Feb. 4, 2025, to include a vaccine expert insight.
After a record-setting 2024, São Paulo state in Brazil is on pace to report a significant Dengue virus outbreak in 2025.
In the first month of 2025, São Paulo's Dengue dashboard shows over 108,000 cases and 25 related fatalities.
The state of São Paulo is a tourist hot-spot, welcoming over 6 million visitors last year.
Nationally, the Pan American Helath Organization reported Brazil had confirmed about 194,000 probable Dengue cases in January 2025.
The U.S. CDC says that Dengue is a year-round risk in various countries in the Region of the Americas. And the agency has identified a higher-than-expected number of Dengue cases among U.S. travelers returning from those countries.
To alert international travelers to this health risk, the CDC republished a Level 1 Travel Health Advisory in 2024, identifying 12 countries in the Americas reporting Dengue outbreaks. The CDC strongly recommends avoiding mosquito bites when visiting Dengue-endemic areas in Brazil.
While other countries have approved a second-generation Dengue vaccine in 2025, the U.S. has not.
The Pan American Health Organization (PAHO) has confirmed local transmission of the Zika virus in countries and territories in the Region of the Americas for several years.
As of February 1, 2025, 303 Zika cases were reported in the Americas this year, with cases reported in Brazil, Bolivia, and Colombia.
Last year, the PAHO reported a 13% increase in cases.
Over 42,127 ZIka cases and two related fatalities in the Americas in 2024.
In 2024, very few cases were reported in the United States. The CDC's preliminary data show 19 travel-related cases and nine locally acquired cases in U.S. territories, such as Puerto Rico.
While the CDC does not recommend any Zika vaccine candidate, Valneva SE's VLA1601 second-generation purified, inactivated, whole Zika virus vaccine candidate is the most advanced Zika vaccine in development. However, this innovative vaccine will probably not be commercially available in 2025.
During the summer of 2024, the Pan American Health Organization (PAHO) issued several alerts that revealed a spike in Oropouche disease cases, including fatalities, in the Region of the Americas.
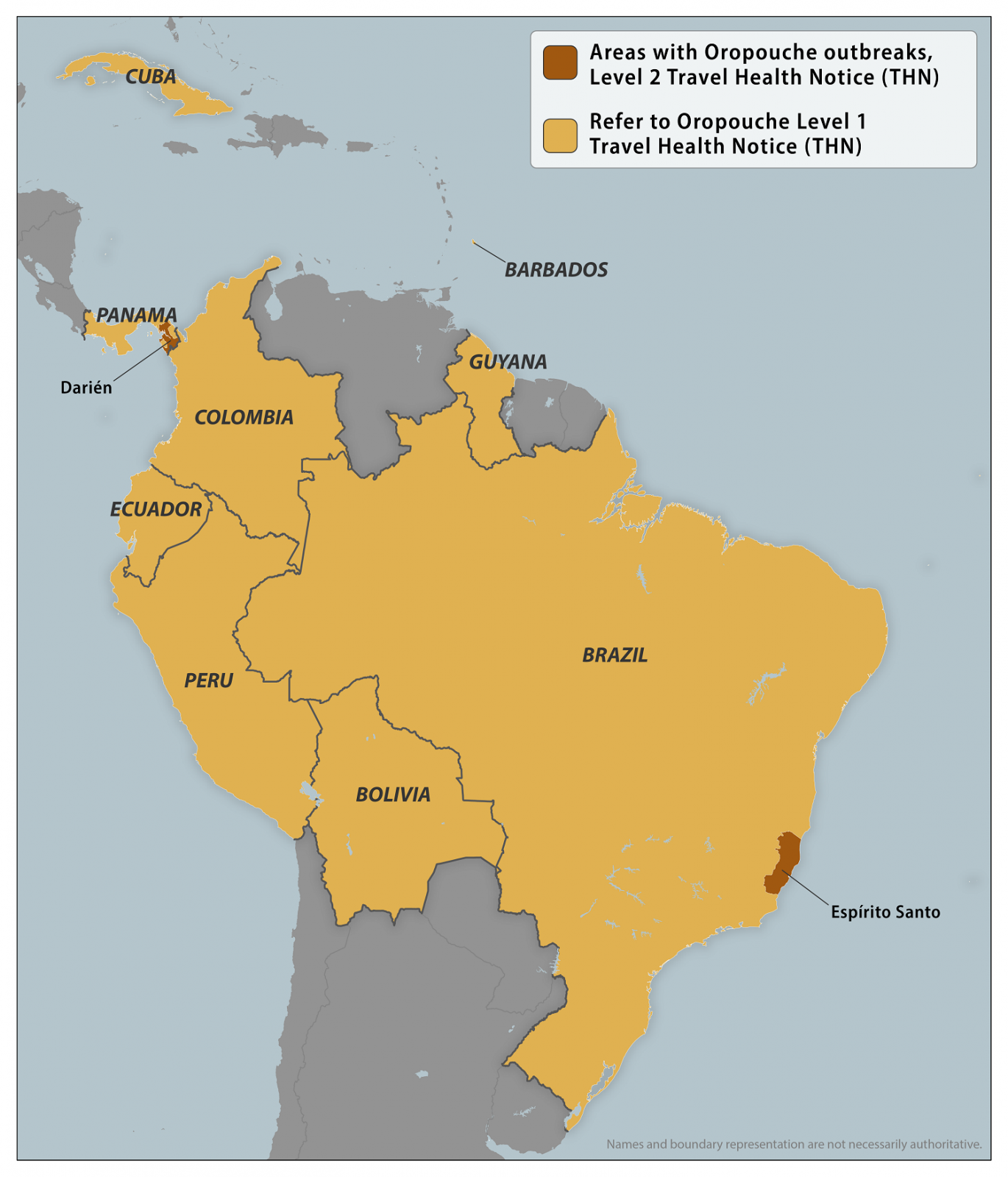
As of January 31, 2025, the U.S. CDC confirmed recent outbreaks of Oropouche in Espírito Santo, Brazil, and Darién Province, Panama. To notify travelers of this health risk, the CDC issued a Level 2 Travel Health Notice.
It says all travelers to these areas should prevent bug bites during travel to protect themselves from infection. They should also prevent bug bites for 3 weeks after travel to avoid possibly spreading the virus to others if they are in areas where mosquitoes and biting midges are active.
Furthermore, healthcare providers should inform women who are pregnant and considering travel to areas with reported Oropouche virus transmission of the possible risks to the fetus. If a pregnant woman decides to travel, counsel her to prevent bug bites.
This new Travel Health Notice follows a Level 1 alert issued in 2024.
The CDC says there is no evidence of Oropouche virus transmission in the United States, but various states (Florida) have reported cases detected in international travelers.
In 2024, 108 neuroinvasive and non-neuroinvasive Oropouche cases were reported in six U.S. jurisdictions. The CDC also says that the extent to which the Oropouche virus could spread in Puerto Rico and the U.S. Virgin Islands is unknown.
From a prevention perspective, since no approved vaccines are available in February 2025, avoiding bug bites is a person's best option.