Search API
The U.S. National Institute of Allergy and Infectious Diseases has initiated a Phase 1 clinical trial for a new ferritin-based nanoparticle vaccine aimed at preventing infection by Epstein-Barr virus (EBV).
Since May 2025, this study has been conducted at the National Institutes of Health Clinical Center in Maryland, and began to evaluate the safety of a 3-dose vaccination regimen of an adjuvanted EBV gH/gL/gp42-ferritin nanoparticle vaccine with or without gp350-ferritin, with 750 participants.
This represents a significant milestone in the development of an EBV vaccine, as there is currently no FDA-approved vaccine available for this virus.
The U.S. CDC states most people will get infected with EBV in their lifetime, especially in childhood, and will not have symptoms. EBV is known to cause infectious mononucleosis and has been associated with several autoimmune diseases and cancers.
According to a NIH media release in 2022, "A vaccine that could prevent or reduce the severity of infection with the Epstein-Barr virus could reduce the incidence of infectious mononucleosis and might also reduce the incidence of EBV-associated malignancies and autoimmune diseases," said NIAID Director Anthony S. Fauci, M.D.
According to ClinicalTrials.gov, this study's expected completion date is October 2027.

The U.S. CDC reported today that the number of acute respiratory illnesses causing people to seek healthcare is at a very low level nationwide.
However, Respiratory Syncytial Virus (RSV) is a prevalent respiratory virus that is typically found first in Florida each year.
The CDC says young children face a higher risk of severe illness due to RSV.
Recent Florida Department of Health data show that Florida's RSV season lasts longer than in other parts of the country and exhibits unique regional patterns.
As of August 9, 2025, Florida reported there have been a total of 22 RSV outbreaks in the current season, with zero new outbreaks in the current week. Florida's Central Region, which includes Orlando, has reported 13 of these outbreaks this year.
Florida's health department notes that identifying unique seasonal and geographic trends in RSV activity in Florida has significant implications for prescribing patterns and initiating prophylaxis in children.
Additionally, the American Academy of Pediatrics (AAP) currently recommends that pre-approval for prophylactic treatment be made based on state surveillance data.
The CDC and AAP say RSV monoclonal antibody (mAb) therapy prevents serious lower respiratory tract disease (LRTD) caused by RSV in newborns and young children during their first RSV season.
A CDC report released on May 8, 2025, demonstrated that in 2024–25, RSV hospitalization rates were 45-52% lower in infants younger than 3 months old and 28-43% lower in infants younger than 8 months old who received an approved mAB compared to the 2018-2020 seasons before product introduction.
Both of these health agencies recommended that new mothers speak with a healthcare provider about immunization options before exposing an infant to RSV.
In addition to RSV, Florida has reported numerious chikungunya, dengue, and malaria cases in 2025.

In the second quarter of 2025, the World Health Organization (WHO) reaffirmed that poliovirus continues to pose a global public health emergency.
However, significant progress has been made in the fight against polio within the African Region.
On August 15, 2025, WHO Africa reported that various cross-border initiatives, including vaccination campaigns, are paving the way for a future free of polio for millions of children across the continent.
Across the Lake Chad Basin and Sahel, countries are synchronizing immunization campaigns to tackle polio in one of Africa's most challenging regions. About 161 million children were vaccinated from April to June.
Similarly, Kenya, Ethiopia, and Somalia vaccinated over 18 million children in a synchronized effort in April 2025.
Just off Africa's east coast, Madagascar also marked a significant milestone this quarter with the official declaration of the closure of the circulating variant type 1 polio outbreak in May. Following a comprehensive Outbreak Response Assessment, the country successfully halted transmission of the virus, demonstrating the effectiveness of robust response strategies that included extensive vaccination rounds and enhanced surveillance.
In Benin, a recent nationwide vaccination campaign successfully reached nearly 2.5 million children. The campaign even extended to remote areas, showcasing Benin's resilience and determination in its response to the threat of polio.
Meanwhile, Malawi is strengthening its healthcare infrastructure with investments in cold chain improvements, workforce training, and community engagement—ensuring that its health systems are ready to prevent future outbreaks, added the WHO.
For an overall perspective, the WHO published a scorecard with key indicators concerning outbreak response, surveillance, and polio transition activities in the African Region.
As of August 16, 2025, the WHO and the U.S. CDC recommend that people complete the primary polio vaccination series and, for some adults, consider a booster dose before visiting poliovirus outbreak areas.
Based on recent news, novel polio vaccines may soon become available that reduce poliovirus shedding and polio cases.
When departing abroad from the United States, various travel vaccine clinics and pharmacies offer polio vaccination services.

Chikungunya is a mosquito-borne infection that affects 100 countries worldwide as of 2025. Many international travelers returning to England, Wales, and Northern Ireland from these locations become infected with this severe virus.
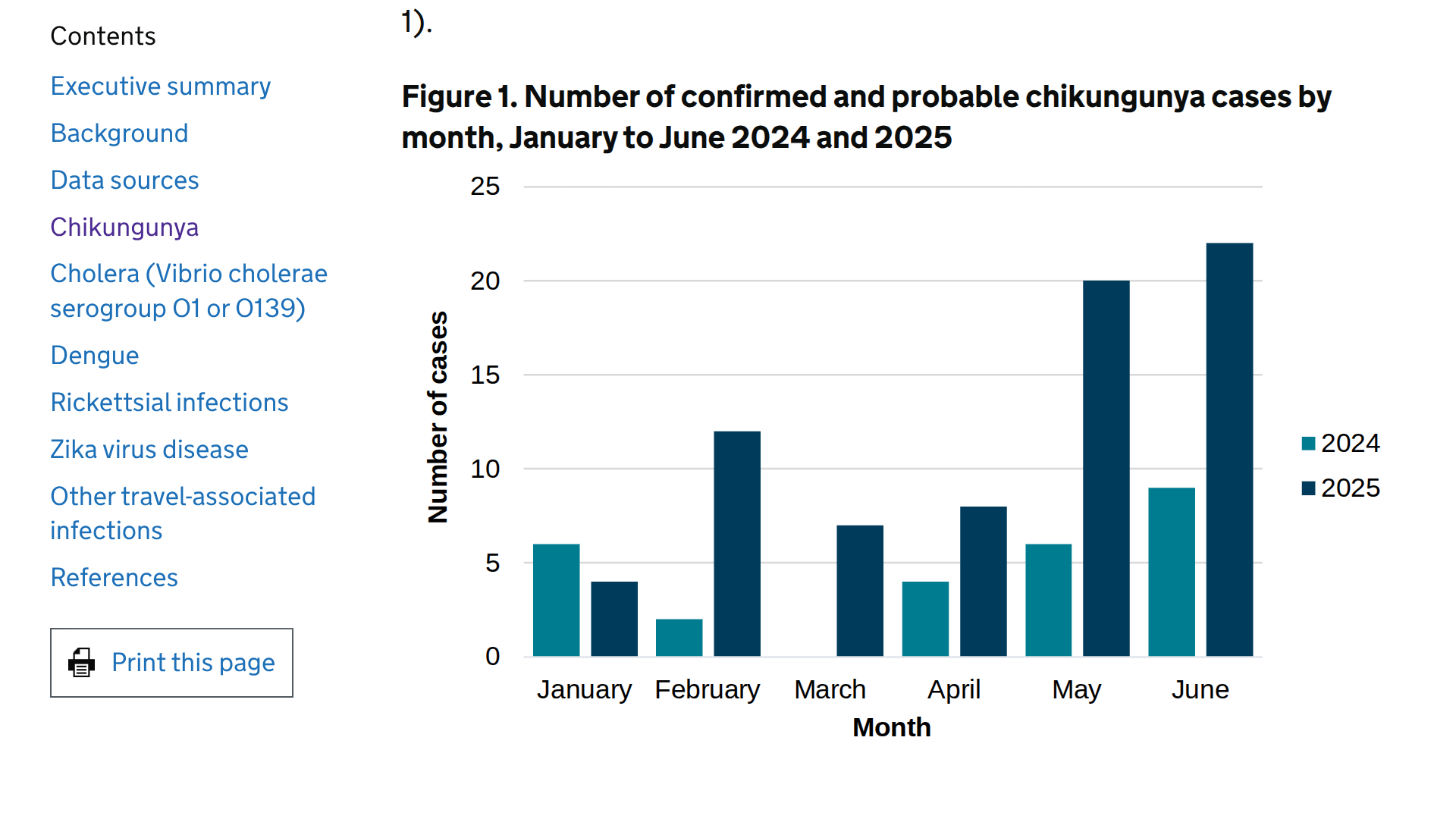
According to the latest data from the UK Health Security Agency (UKHSA), published on August 14, 2025, there has been an increase in travel-associated chikungunya cases in the United Kingdom.
A total of 73 cases were reported between January and June 2025, compared to just 27 cases during the same period in 2024.
This marks the highest number of cases recorded for this period to date, says the UKHSA.
Most cases reporting travel to Sri Lanka, India, and Mauritius align with ongoing local outbreaks in countries in the Indian Ocean region.
In the UK, chikungunya vaccines were approved for most adults in 2025.
AstraZeneca has launched an innovative at-home delivery service for FLUMIST®, an intranasal influenza vaccine.
According to a press release on August 15, 2025, after verifying eligibility and insurance, FLUMIST will be prescribed and shipped directly to the consumer's home on their chosen date.
The package will include clear administration instructions, storage guidance, and disposal information.
FLUMIST is the first and only seasonal influenza vaccine approved for self-administration by adults aged 18 to 49. A parent or caregiver can also administer it to individuals aged 2 to 17.
Initially approved by the Food and Drug Administration in 2003, eligible individuals and their families can now conveniently protect themselves during the 2025-26 flu season from the comfort of their homes.
Joris Silon, US Country President and Senior Vice President at AstraZeneca, stated in a press release, "The launch of FluMist Home marks a transformational moment in the evolution of influenza protection, providing a simple and accessible option directly to consumers."
He added, "FluMist Home reflects the growing importance of direct-to-consumer offerings and underscores our commitment to continuous innovation, making it easier for people to get vaccinated and stay protected."
Individuals aged 18 and older can visit www.FluMist.com to learn more and place an order. They will be directed to complete a medical screening questionnaire, which a licensed healthcare provider will review to determine eligibility.

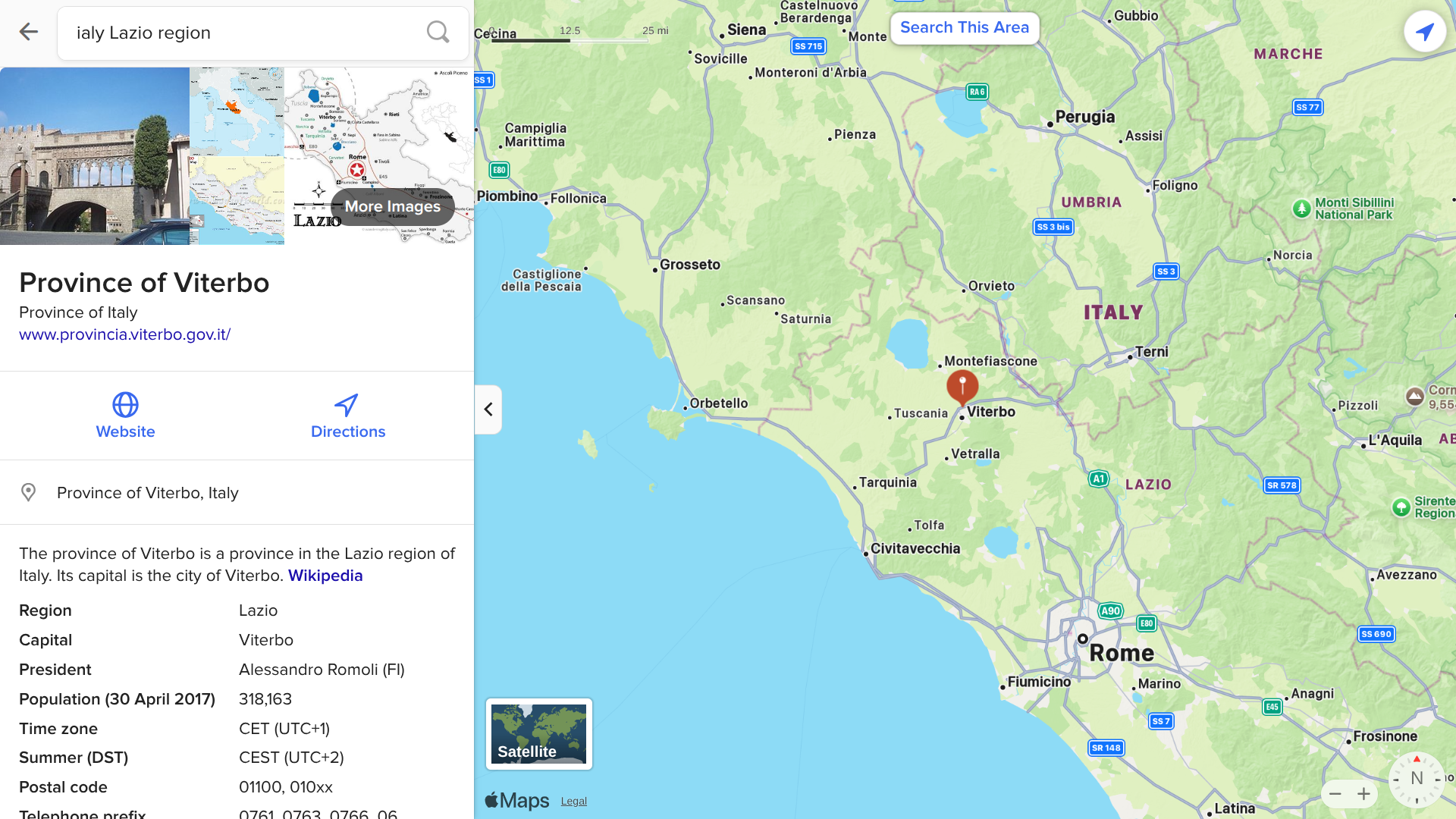
While West Nile virus (WNV) has been determined to be endemic throughout most of Italy, the current outbreak has been centered in the Lazio Region, one of the 20 administrative regions. Situated in the central peninsular area, Lazio has over 5.7 million inhabitants.
According to the health ministry Bollettino N. 5, Lazio has reported 140 WNV cases as of August 14, 2025.
Overall, a total of 275 cases of WNV infection, including 19 related fatalities, have now been confirmed in Italy since the beginning of 2025.
Local media reported in early August 2025, the health ministry has said the situation is under control and there is no need for alarm, as while areas of Italy previously unaffected have been hit, the contagion and fatality figures are in line with those of recent years.
In 2022, human cases were reported in 25 provinces (128 municipalities) of the regions Emilia-Romagna, Friuli-Venezia Giulia, Lombardia, Piemonte, Sardegna, Toscana, and Veneto.
Since the beginning of 2025, five other European countries have reported human cases: Bulgaria, France, Greece, Hungary, and Romania.
Currently, there are no vaccines approved to prevent WNV cases.
After decades of progress against cholera outbreaks, cases are again on the rise, even in countries that had not seen the disease in years. And with a multi-year vaccine shortage, health leaders were confronted with significant challenges.
According to the World Health Organization (WHO), 44 countries reported cholera cases in 2022, a 25% increase from the 35 countries that reported cases in 2021.
On August 15, 2025, Kathryn Alberti, WHO Technical Officer for cholera, provided an update on the global cholera situation in a media release.
This year, more than 390,723 cholera cases and 4,332 related fatalities have been reported across 31 countries. These figures are underestimates, says Alberti.
Fortunately, the cholera vaccine available has recently improved.
Since December 2024, oral cholera vaccine production has hit record levels - 6 million doses monthly, the highest since 2013. But record production has been outpaced by record demand.
Since January 2025, the International Coordinating Group on Vaccine Provision (ICG) has received 38 requests from 12 countries—triple the number compared to the same period last year.
Already this year, over 40 million doses have been allocated by the ICG, compared to 35 million doses allocated in all of 2024.
More than 85% of approved doses this year are for countries facing humanitarian crises, with the largest share going to the Republic of Sudan.
In Sudan's Darfur region alone, MSF teams saw over 2,300 cholera patients and 40 deaths in early August 2025.
In the United States, travel vaccine clinics and pharmacies continue to report ample cholera vaccine supplies. The WHO and the U.S. CDC recommend vaccination before visiting a cholera outbreak zone in 2025.