Search API
Local media reported that Bahamian Health Minister Michel Darville announced that The Bahamas is preparing to cancel its contracts with Cuban health professionals.
According to Reuters on June 16, 2025, the reason for the cancellation is that negotiations are underway with the US government.
The NGO Archivo Cuba has reported that Cuban specialist medical advisors in the Bahamas were paid $12,000 per month, while biomedical engineers received $$5,000.
As of June 16, 2025, the U.S. CDC says 'check the vaccines, such as measles or typhoid, and medicines list, and visit your doctor at least a month before your trip to The Bahamas.
Previously, the U.S. and Canadian governments issued travel advisories regarding jet ski activities in the Bahamas.
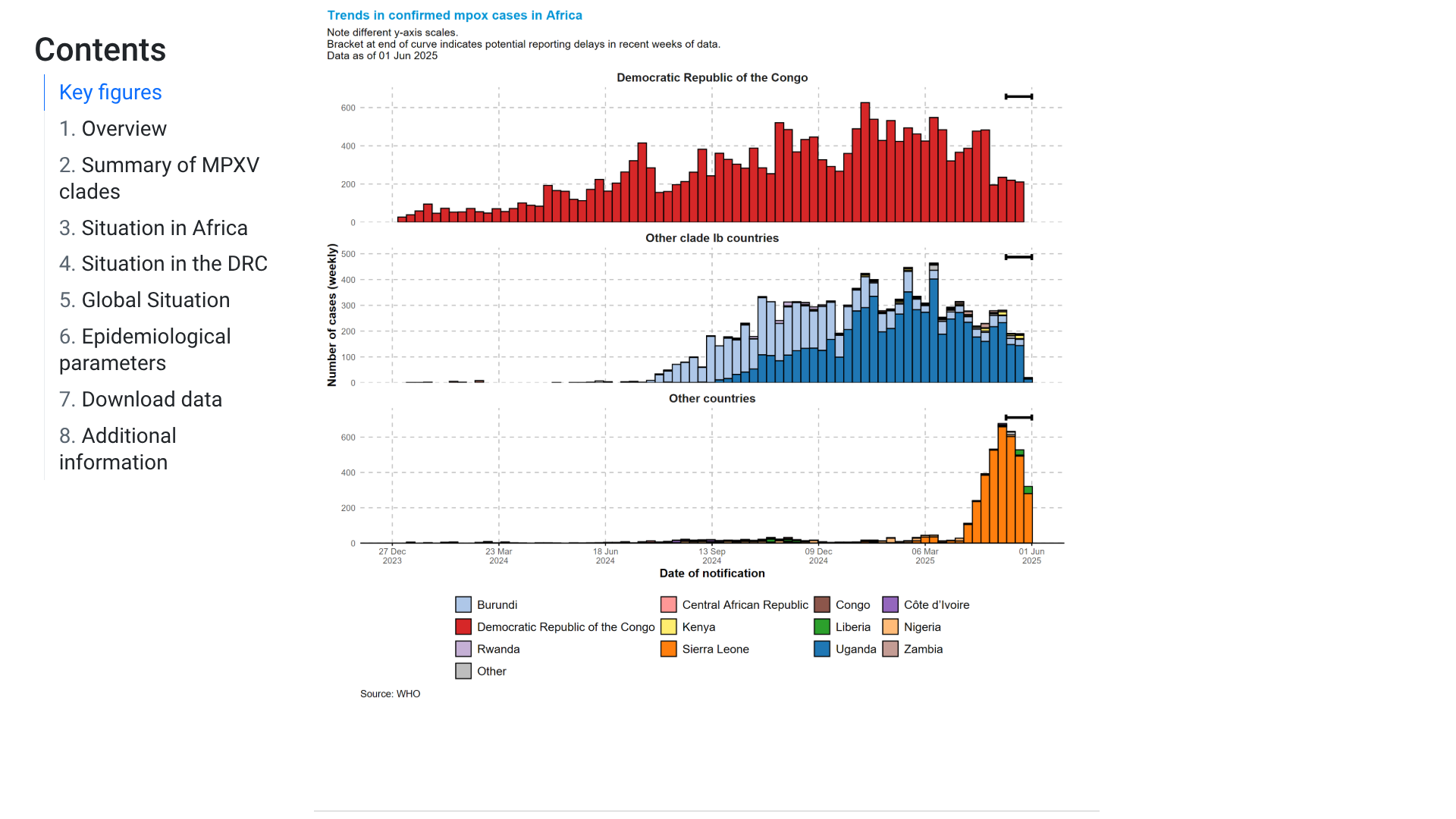
The World Health Organization (WHO) Director-General, Dr Tedros Adhanom Ghebreyesus, today announced that the mpox upsurge continues to meet the criteria of a public health emergency of international concern (PHEIC).
As of June 9, 2025, this PHEIC has been declared, based on the continuing rise in the number of cases, including a recent increase in West Africa, and likely ongoing undetected monkeypox virus (MPXV) transmission in some countries beyond the African continent.
The Director-General also concurred with and issued the Committee's revised temporary recommendations to Member States experiencing mpox outbreaks.
Regarding preventive vaccinations, the WHO advises preparing for and implementing targeted use of vaccines for "Phase 1- Stop the outbreak" through the identification of disease hotspots and targeting those groups at high risk of mpox exposure to interrupt sustained community transmission.
As of early June 2025, the U.S. CDC states that JYNNEOS is a two-dose vaccine developed to protect against mpox and smallpox. People need to receive both doses of the vaccine for optimal protection against mpox.
In the United States, JYNNEOS® is commercially offered at health clinics and pharmacies, with insurance options available.
Furthermore, to be most effective, mpox vaccination should be included as part of broader prevention activities and routine sexual health care, such as HIV or gonorrhea.
'Whether or not you've been vaccinated, continue to reduce your risk of getting mpox,' writes the CDC.
Through the initial five months of 2025, Zika virus (ZIKV) outbreaks continue as a significant, measurable public health concern worldwide.
In the Region of the Americas, over 12,600 Zika patients have been identified as of June 1, 2025.
Last year, 42,127 ZIka cases and two related fatalities were reported in the Americas in 2024, led by Argentina, Brazil, Bolivia, Colombia.
Foremost among public health leaders' focus is when a pregnant woman becomes infected with this mosquito-transmitted virus. While pregnant, ZIKV can induce severe defects of the fetal brain and, eventually, microcephaly in the infant.
To better understand this health risk, an Ohio State University (OSU) study published in the Proceedings of the National Academy of Sciences on May 23, 2025, reveals the biological secret to the Zika virus's infectious success.
These researchers found that Zika utilizes the host cells' own "self-care" system to clear away useless molecules, thereby suppressing the host proteins that the virus has employed to enter those cells in the first place.
They wrote in a press release on May 27, 2025, 'While these cell surface proteins are valuable for viral entry, they also have roles in producing an antiviral response. Before that can happen, the virus manipulates a process cells use to keep themselves healthy to lower the proteins' activity, clearing the way for unfettered viral infection.'
'Though other viruses, such as HIV, are known to silence host receptors that let them into cells, Zika is unusual for having at least three of its proteins that can get the job done,' said Shan-Lu Liu, senior author the study and a virology professor in the Department of Veterinary Biosciences at OSU.
"That's the most interesting part: It's amazing that not only one, but several Zika proteins can do this."
"We looked at two Zika virus strains and examined three physiologically relevant cell types. With both strains, we observed downregulation in all three cell types. It looks like this is an important mechanism," added Liu.
Although further research is needed to confirm this, there is a possibility that this mechanism is relevant to the Ebola virus, which utilizes the TIM-1 protein to access host cells, or to other pathogens in the same flavivirus family, including Zika, West Nile, yellow fever, and dengue viruses.
"The bottom line is this speaks to the co-evolution of viral-host interactions. The more important a host factor is to a virus, the more a virus is going to do to take control of it," Liu said. "Understanding these mechanisms is an important part of being prepared for emerging or reemerging viruses that cause infectious diseases."
As of June 2025, there are no Zika preventive vaccines available, and the U.S. CDC recommends pregnant women avoid visiting areas reporting Zika outbreaks.
Over the last few years, Zika cases have been reported in Puerto Rico, Costa Rica, and other tourist favorite destinations.

Thanks to the world's first vaccination program against a leading sexually transmitted infection (STI), thousands of gonorrhoea cases in the United Kingdom could be prevented over the next decade.
The UK's NHS and local government announced on May 21, 2025, that they are launching a vaccine program to prevent the recent increase in gonorrhoea cases. Those who receive the meningococcal B disease vaccine 4CMenB (Bexsero®) could be protected from gonorrhoea by up to 40%.
In 2023, there were 85,000 gonorrhoea diagnoses in England, 300% higher than in 2012.
Eligible patients will be offered the vaccine through local authority-commissioned sexual health services from early August 2025.
Eligible people will also be offered mpox (JYNNEOS), hepatitis A and B, and human papillomavirus vaccinations when attending their appointment for the gonorrhoea vaccine.
James Woolgar, Chair of English HIV and Sexual Health Commissioners’ Group, commented in a press release, “Introducing the world’s first gonorrhoea vaccine programme into England’s sexual health services is a major milestone for public health."
In the United States, Bexsero is FDA-approved, recommended by the CDC for certain people, and available at most clinics and pharmacies.

While this respiratory disease is generally related to camel interactions in the Kingdom of Saudi Arabia, clinical efforts to produce a Middle East Respiratory Syndrome (MERS) vaccine have been elusive.
Since April 2012 and as of mid-March 2025, six World Health Organization regions have reported 2,618 cases of MERS, including 945 deaths, a significant case-fatality rate.
To address this need, CEPI announced, on March 25, 2025, a $2.6 million investment in moving a promising vaccine candidate into preclinical trials.
This new investment, developed by Newark, DE-based Uvax Bio, an early-stage vaccine technology company spun out of The Scripps Research Institute, is based on proprietary protein nanoparticle technology, 1c-SApNP®, licensed from Scripps Research.
The technology is already being tested against other infectious diseases, including HIV, where an in-human trial is ongoing.
Dr. Kent Kester, Executive Director of Vaccine R&D, CEPI, commented in a press release, “Uvax Bio’s unique vaccine could help strengthen our response to future MERS outbreaks while informing us of the vaccines being developed against other coronaviruses.”
Uvax’s novel vaccine design uses tiny protein “nanoparticles” to closely resemble or mimic the size and shape of the MERS coronavirus.
Uvax Bio has analyzed viral structures and designed the technology to present enhanced antigens —parts of the virus that trigger an immune response—in a multilayered scaffold layout. This design offers stability and allows for as many as twenty antigens to be presented at once, which could help provide strong protection by generating both antibody and T-cell immunity.
The 1c-SApNP® technology is also unique as it has been combined with a process called ‘glycan trimming.’
Here, sugar molecules—called glycans—that would generally cover the MERS virus are shortened in the nanoparticle virus-mimicking vaccine design. This could expose additional sites on the antigen surface, enhancing the immune response.
In addition to the vaccine candidate, several MERS vaccines are in clinical development addressing this zoonotic disease with an unknown source in 2025.

Since the Mpox virus swept around the world in May 2022, Germany's Standing Commission on Vaccination has recommended that people at an elevated risk of infection receive a preventive vaccination.
After millions of JYNNEOS® (MVA-BN®, IMVAMUNE®) doses were administered, an observational study published positive effectiveness data today.
The Lancet Infectious Diseases published results from a study conducted at Charité – Universitätsmedizin Berlin on March 18, 2025, that found one dose of the JYNNEOS was 84% in people without HIV and 58% effective against mpox infection overall.
However, due to the significant drop in Mpox infections in the second half of 2022, the study could not determine the additional effect of a second vaccine dose.
Furthermore, Breakthrough infections were associated with reduced symptoms, compared with infections in unvaccinated individuals.
In a related press release, Prof. Leif Erik Sander, Director of the Department of Infectious Diseases and Critical Care Medicine at Charité and a research group leader at the Berlin Institute of Health at Charité, stated, "Our results confirm that a single dose of the vaccine provides good protection against Mpox, at least for a short time."
"That is a very good figure, which is likely increased further by the second vaccine dose."
"The reason is that developing immune protection after vaccination presumably requires specific immune cells called T cells. These T cells often appear at lower levels in people with HIV and are not fully functional, which translates to a weaker immune response. This also corresponds to our observation that these participants experienced fewer local and systemic side effects after receiving the vaccine."
"We assume that people living with HIV develop protection against Mpox after the second vaccine dose, and urgently advise these people to receive the two vaccine doses."
"The immune system typically develops longer-lasting immune protection when exposed to the vaccine on more than one occasion."
Further studies will be required to determine the precise extent of the protective effect in different groups following two vaccine doses.
As of March 29, 2025, the JYNNEOS vaccine is commercially available at many clinics and pharmacies in the United States.