Search API
When Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) was founded in 2016, the early-stage antibiotic pipeline was stalled.
Since its inception, CARB-X has supported 115 R&D projects in 14 countries, and CARB-X product developers have made significant progress.
Recently, CARB-X awarded Baxiva AG $3 million to develop its multivalent glycoconjugate vaccine to prevent extraintestinal pathogenic Escherichia coli (ExPEC) infections.
Baxiva's proprietary conjugation platform streamlines the development of multivalent vaccines targeting the serotype-specific polysaccharides of Gram-negative bacteria, including capsule and O antigens.
The multivalent vaccine targets the most common serotypes associated with invasive ExPEC infections.
Multivalent vaccines are designed to prevent infections caused by multiple strains or types of a single pathogen. The glycoconjugate formulation combines polysaccharide (sugar) from a pathogen's surface with a carrier protein to enhance immune response and therefore the effectiveness of the vaccine.
Escherichia coli is the leading cause of urinary tract infections, a frequent cause of neonatal sepsis, and is among the leading causes of antimicrobial resistance-associated deaths globally.
"Vaccines are a powerful tool in the global effort to prevent infections and curb the spread of antimicrobial resistance," said Erin Duffy, PhD, R&D Chief of CARB-X, in a press release on August 21, 2025.
"Baxiva's multivalent glycoconjugate vaccine project explores a range of novel polysaccharide antigens in vaccine candidate solutions, addressing a critical unmet need in infection prevention."
"We are excited to welcome Baxiva into the CARB-X portfolio and support the advancement of their platform."
The E. coli bacteria cause most urinary tract infections (UTIs).
As of August 2025, UTI vaccines are unavailable in the United States.

Since 2016, there has been an increase in the number of hepatitis A cases, primarily affecting men in low-endemic countries across Europe.
According to a recent report from the European Centre for Disease Prevention and Control (ECDC), Austria, Czechia, Hungary, and Slovakia reported higher-than-expected numbers of hepatitis A virus (HAV) subgenotype IB cases in 2025.
Additionally, France recently reported HAV cases, with 73 reported in the Rhône department as of August 19, 2025.
This French health ministry HAV data marks a 356% increase in cases compared to the same period in 2024.
The Rhône department is located in the east-central administrative region of Auvergne-Rhône-Alpes, which includes the Lyon Metropolis, and has a population of approximately 1,875,747.
Previous HAV outbreaks in France, particularly in the Seine-Maritime department, are reported by the ECDC.
Since HepA is a vaccine-preventable disease, the U.S. CDC recommends hepatitis A vaccination for most international travelers engaging in higher-risk activities, such as visiting smaller cities, villages, or rural areas where they may be exposed to food or water contamination.
And for travelers who plan on eating street food in France.
In the United States, HepA vaccines are generally offered at travel pharmacies and clinics in 2025.
In June 2025, the World Health Organization (WHO) renewed its global alert regarding the poliovirus emergency, highlighting several countries facing significant health risks.
According to a WHO Disease Outbreak News published on August 20, 205, Israel is confronting an unquantified health risk that has been detected in Jerusalem and the Central Region.
On August 4, 2025, Israel notified WHO of a circulating vaccine-derived poliovirus type 1 (cVDPV1) outbreak.
Between February and July 2025, nine genetically linked virus isolates were found in environmental samples from seven sites. However, no human cases of paralytic polio have been reported.
Vaccine-derived poliovirus is a well-documented strain of poliovirus mutated from the strain contained initially in oral polio vaccines.
The WHO stated Israel discontinued routine use of the bivalent oral polio vaccine in March 2025 but continues to administer four doses of inactivated polio vaccine (IPV) as part of the routine immunization schedule up to 12 months of age.
The WHO/UNICEF Estimates of National Immunization Coverage for three doses of IPV in 2024 were 98%.
Unfortunately, polio vaccination coverage in Jerusalem is notably lower and below the WHO's recommended coverage threshold, which is necessary to maintain sufficient population immunity and prevent poliovirus transmission between people.
The WHO currently assesses the risk of international spread associated with this cVDPV1 detection as low due to high overall population immunity, robust poliovirus surveillance, and response capacity.
To alert international travelers visiting Israel, the U.S. CDC's updated Level 2 - Practice Enhanced Precautions, Travel Health Advisory, includes Israel.
The CDC writes, 'Before travel to any destination listed, adults who previously completed the routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine.'
In the United States, the IPV polio vaccine is offered at travel clinics and pharmacies in 2025.
The European Union (EU) and the United States government have announced the details of a Framework on an Agreement on Reciprocal, Fair, and Balanced Trade (Framework Agreement), which includes pharmaceutical products such as vaccines.
The mutual agreement includes 15% tariffs on EU exports to the United States.
The August 21, 2025, statement confirmed that the U.S. and the EU, in line with their relevant internal procedures, will promptly document the Framework Agreement, which should reveal which EU-produced vaccines are subject to this tariff, which may impact the cost to consumers in the U.S.
This pending vaccine list may include high-demand travel vaccines for chikungunya, cholera, and Japanese Encephalitis.

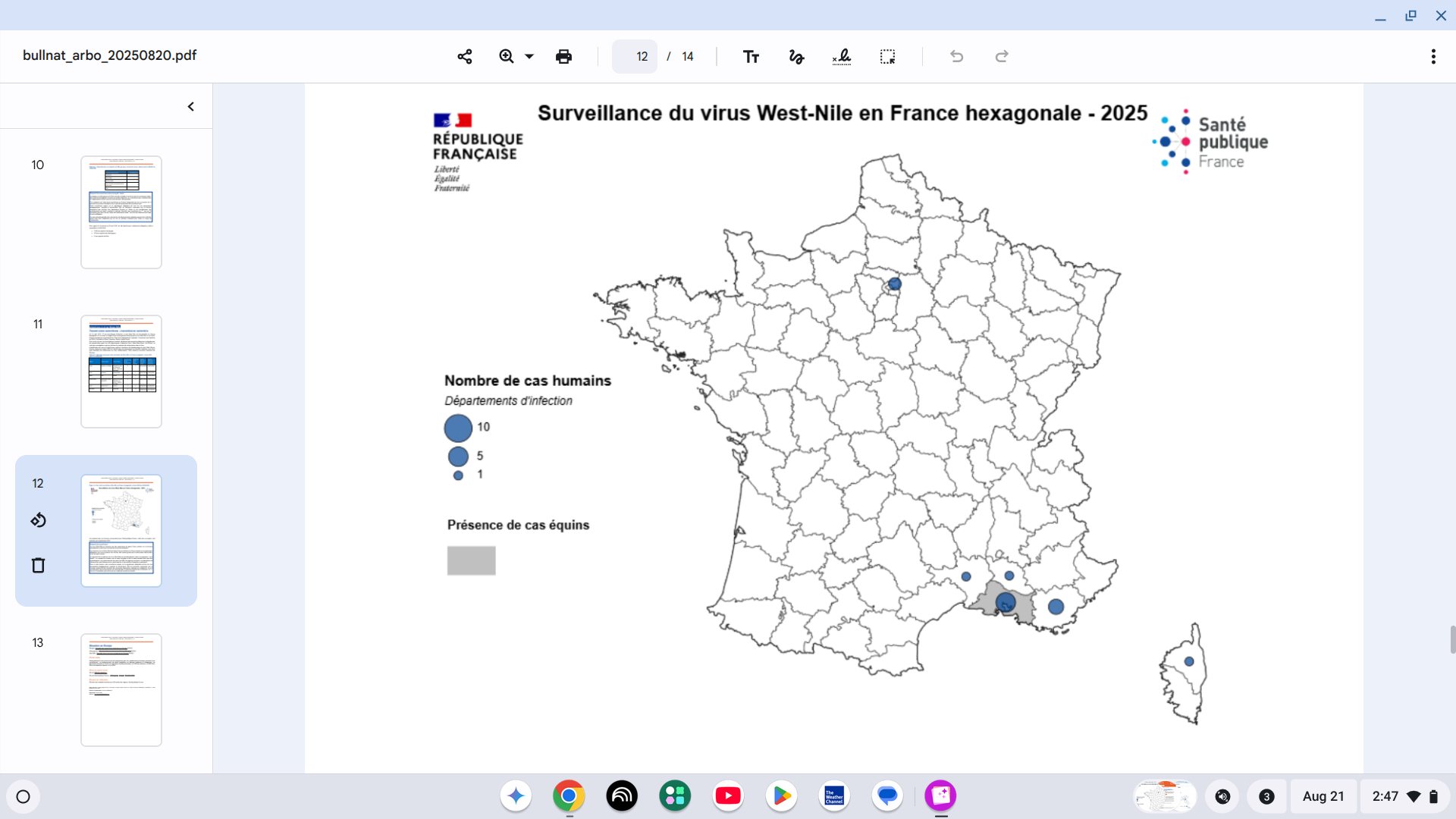
Since the beginning of 2025, the European Centre for Disease Prevention and Control (ECDC) has reported West Nile virus infections in eight countries: Bulgaria, France, Greece, Hungary, Italy, Romania, Serbia, and Spain.
Case numbers reported so far this year are slightly above the average for the past decade, says the ECDC.
However, these figures remain lower than those seen in 2024 and 2018 when West Nile virus (WNV) circulation was particularly intense, with 382 and 385 cases reported by this point in the year, respectively.
While the geographical distribution has been centered in northern Italy, the ECDC's data is now indicating the southern coast of France has become an outbreak zone.
As of August 19, 2025, 13 human cases of vector-borne West Nile virus infection have been identified in six departments of mainland France.
The affected regions are PACA, Occitanie, Corsica, and, for the first time, Île-de-France.
As the summer holiday season ends in 2025, the hospitalisation rate has been notably high, with 100% of reported cases requiring hospitalization this year, compared to 93% over the past decade, according to the ECDC.
The case fatality rate so far this year stands at 7%, which is similar to the 11% observed in the previous decade.
Additionally, neurological manifestations have been reported in 56% of cases this year, compared to 66% in the past decade. Generally, cases with more severe symptoms are more likely to be diagnosed, leading to an expectation of a dominance of neurological cases.
As of August 21, 2025, the U.S. CDC's Travel Health Advisory for France does not identify WNV as a risk.
Both the ECDC and other health agencies recommend avoiding mosquito bites as the best strategy to reduce this health risk, as there are currently no approved vaccines available.
Europe is experiencing a record number of outbreaks of mosquito-borne illnesses in 2025, including chikungunya.
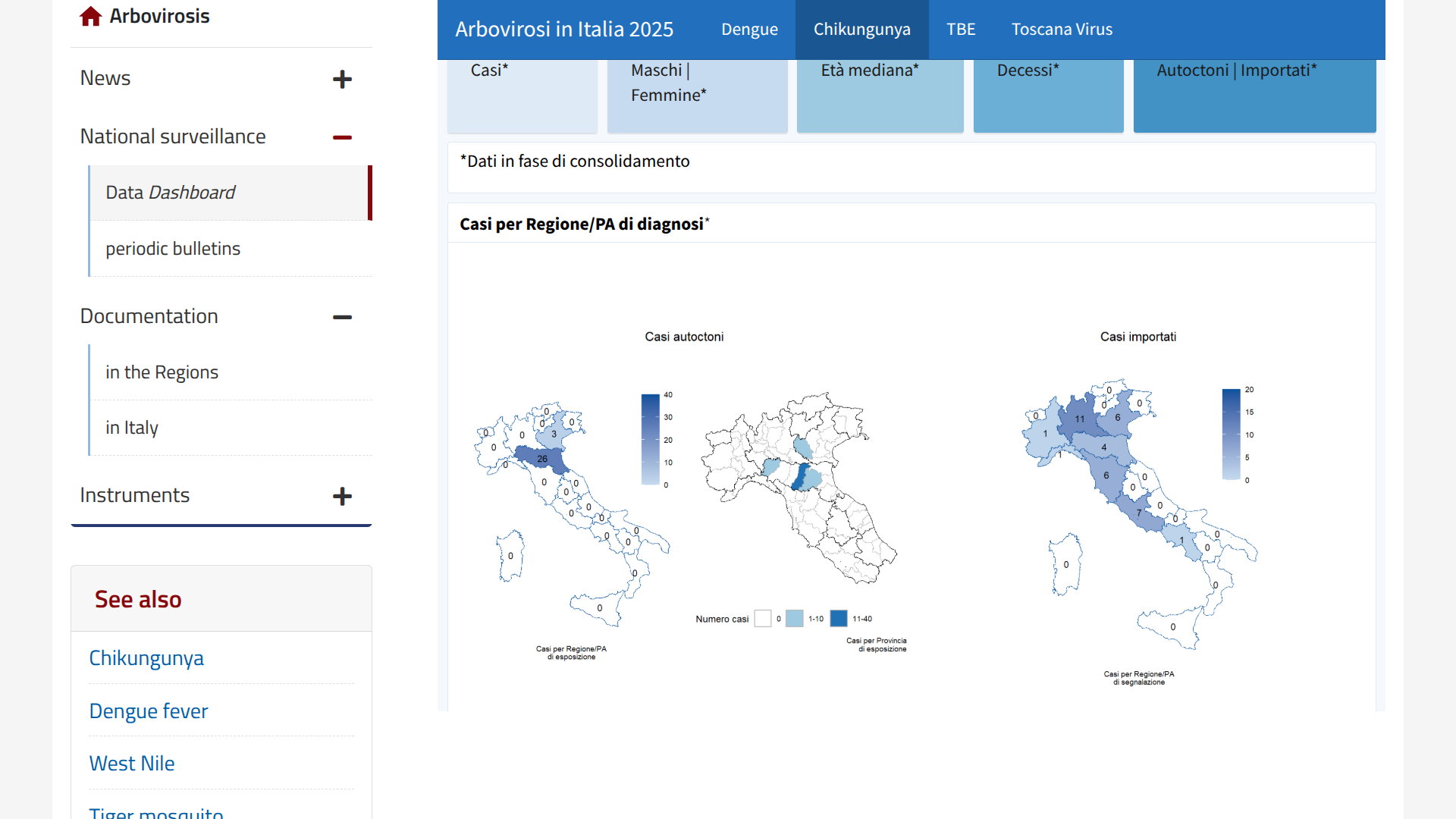
New data published today by the Italian Istituto Superiore di Sanità (ISS) indicates that the country's fight against chikungunya fever continued throughout the early weeks of August 2025.
On August 21, 2025, ISS reported four local transmission events of the chikungunya virus had been identified in the northern regions of Emilia-Romagna and Veneto in two outbreaks.
Chikungunya is a viral disease transmitted to people by virus-carrying mosquitoes, often found in these regions' altitudes, through November each year.
From January to August 19, 2025, Italy's national surveillance system has recorded 66 confirmed cases of chikungunya (37 travel-associated cases and 29 indigenous (local) cases, and no related deaths, with a median age of 53 years.
From a prevention perspective, the U.S. CDC recommends either of the two approved chikungunya vaccines for international travelers visiting outbreak zones in 2025.
These vaccines, and other travel vaccines, are commercially offered at certified clinics and pharmacies in the United States.
As of mid-2025, the Philippines has recorded over 123,000 dengue cases nationwide from January to June, and at least 437 dengue-related deaths, with the majority occurring among children.
While the Philippines Department of Health (DOH) considers the situation manageable, as of August 20, 2025, the data reflects a 7% increase.
In Lapu-Lapu City, Cebu, dengue cases surged by 35% compared to the same period last year. Dr. Vincent Jess Montejo of the City Health Office assured the public that the situation is not alarming.
"It's not an immediate health problem that we should be concerned about… not panic-level," Dr. Montejo said in a Facebook post.
The DOH says the case increase rate has prompted intensified vector control and public awareness campaigns.
Although there is a second-generation dengue vaccine available in numerious countries, the Philippine FDA has not yet approved it, nor the U.S. FDA.
To alert international travelers of this severe health risk, the U.S. CDC included the Philippines in its recent Level 1 Travel Health Advisory.
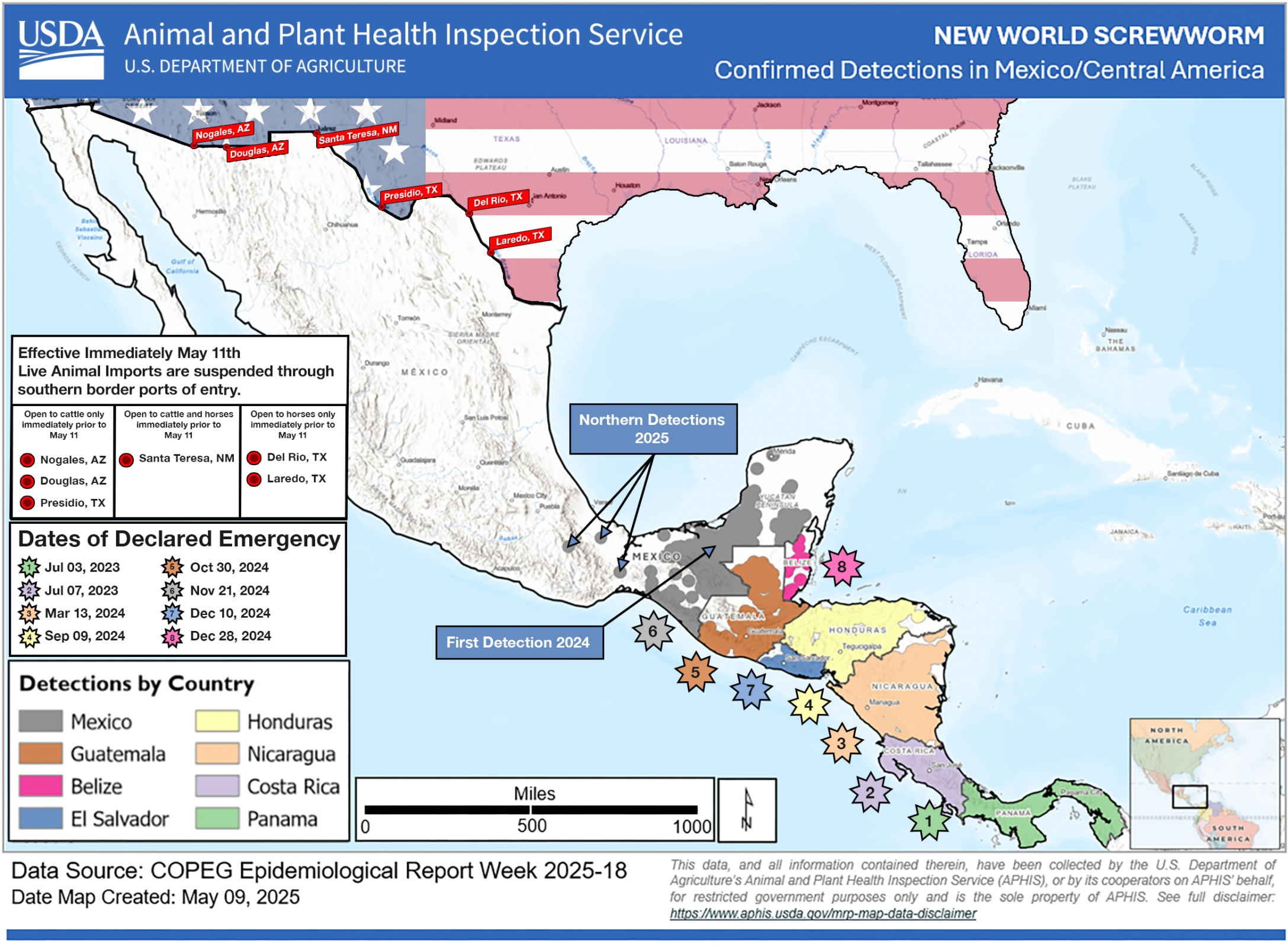
The U.S. Department of Health and Human Services recently issued a declaration that allows the U.S. Food and Drug Administration (FDA) to issue Emergency Use Authorizations for animal drugs to treat or prevent infestations caused by the New World Screwworm (NWS).
As of August 20, 2025, there are no FDA-approved drugs for NWS, nor are there vaccines authorized to protect people in the United States.
As of today, NWS infestations have been confirmed in Central America and Mexico, but not in Texas.
The FDA stated in a press release that the parasite's risk to human health in the United States remains very low. Still, the potential future threat to animal populations and the food supply chain requires proactive action.
“Our priority is to safeguard both animal health and the nation’s food supply,” said FDA Commissioner Marty Makary, M.D., M.P.H., in a press release.
“FDA is acting swiftly and responsibly to help ensure we have the necessary tools to prevent and control New World Screwworm, minimizing risks to agriculture and public health.”
Over the last two months, about $900 million has been committed by the U.S. government to combat the NWS from reaching the U.S.
NWS infests warm-blooded animals, including livestock, pets, wildlife, and, in rare cases, humans, causing severe tissue damage and sometimes death, writes the FDA.
The Florida Department of Health in Hillsborough County (DOH Hillsborough) recently informed residents of a confirmed human case of locally-acquired dengue fever.
According to data published by the state (Week 33) on August 19, 2025, this is the 14th local dengue case reported in 2025. Previous dengue cases have been confirmed in the east coast near Melbourne (11) and Miami (2).
DOH-Hillsborough and Hillsborough County Mosquito Control stated in a media release that they are coordinating surveillance and prevention efforts by conducting aerial spraying to reduce the spread of this mosquito-transmitted virus to other people.
This area of Florida includes cities such as Tampa and has a local population of over 1.4 million.
Additionally, Florida has reported 154 dengue cases among international travelers, many of whom had recently visited Cuba.
As of August 20, 2025, dengue vaccines are not offered in Florida.

