Search API
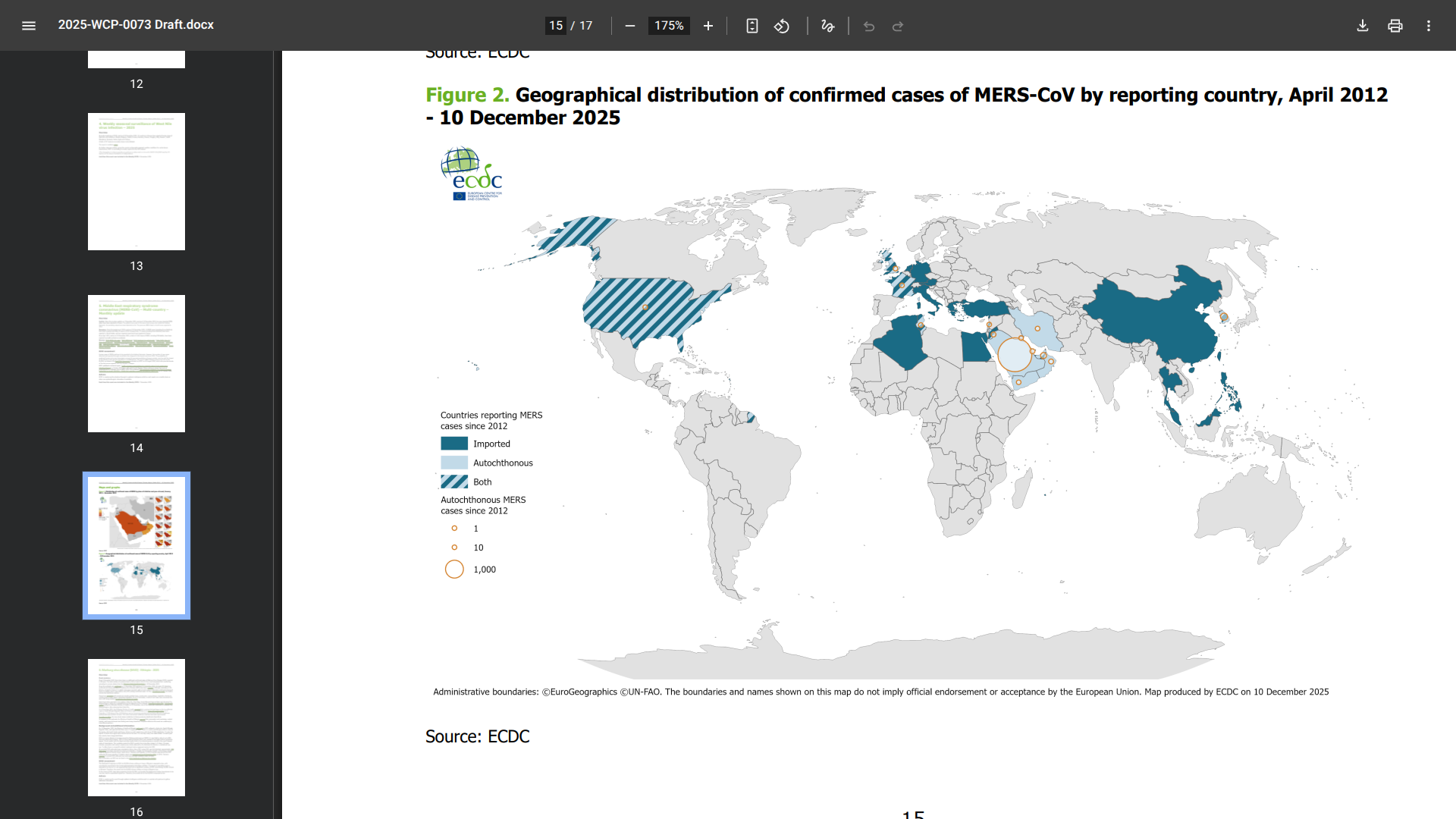
With the initial case of Middle East respiratory syndrome (MERS) detected in 2012 in the Arabian Peninsula, a few international travelers have been diagnosed with this severe disease outside of this region.
Since the beginning of 2025, 14 MERS cases, including three fatalities, have been reported primarily in the Kingdom of Saudi Arabia.
Recently, the European Centre for Disease Prevention and Control (ECDC) confirmed two MERS cases have been reported in France, the first MERS cases in France in 12 years.
The patients are older men treated in hospitals in Lyons and southwest France.
"The patients are being monitored [in hospital] as a precautionary measure, and their conditions are stable," Stéphanie Rist, minister for health, families, and autonomy, said in a media statement on December 5, 2025.
Over the years, MERS infections have been associated with exposure to camels.
The ECDC says the probability of sustained human-to-human transmission among the general population in Europe remains very low, and the impact of the disease in the general population is also considered to be low.
And in the United States, the CDC recommends MERS-CoV testing for persons who meet the MERS-CoV person-under-investigation criteria. In the U.S., the clinical and epidemiologic criteria to guide testing were discussed in May 2025.
Since 2012, a total of 2,627 laboratory-confirmed cases have been reported globally, with 947 associated deaths at a case-fatality ratio of 36%.
From a disease prevention option, the World Health Organization (WHO) and the Kingdom of Saudi Arabia have not approved a MERS-CoV vaccine candidate as of late 2025. The WHO says several candidates are being tested in human clinical trials in 2025.
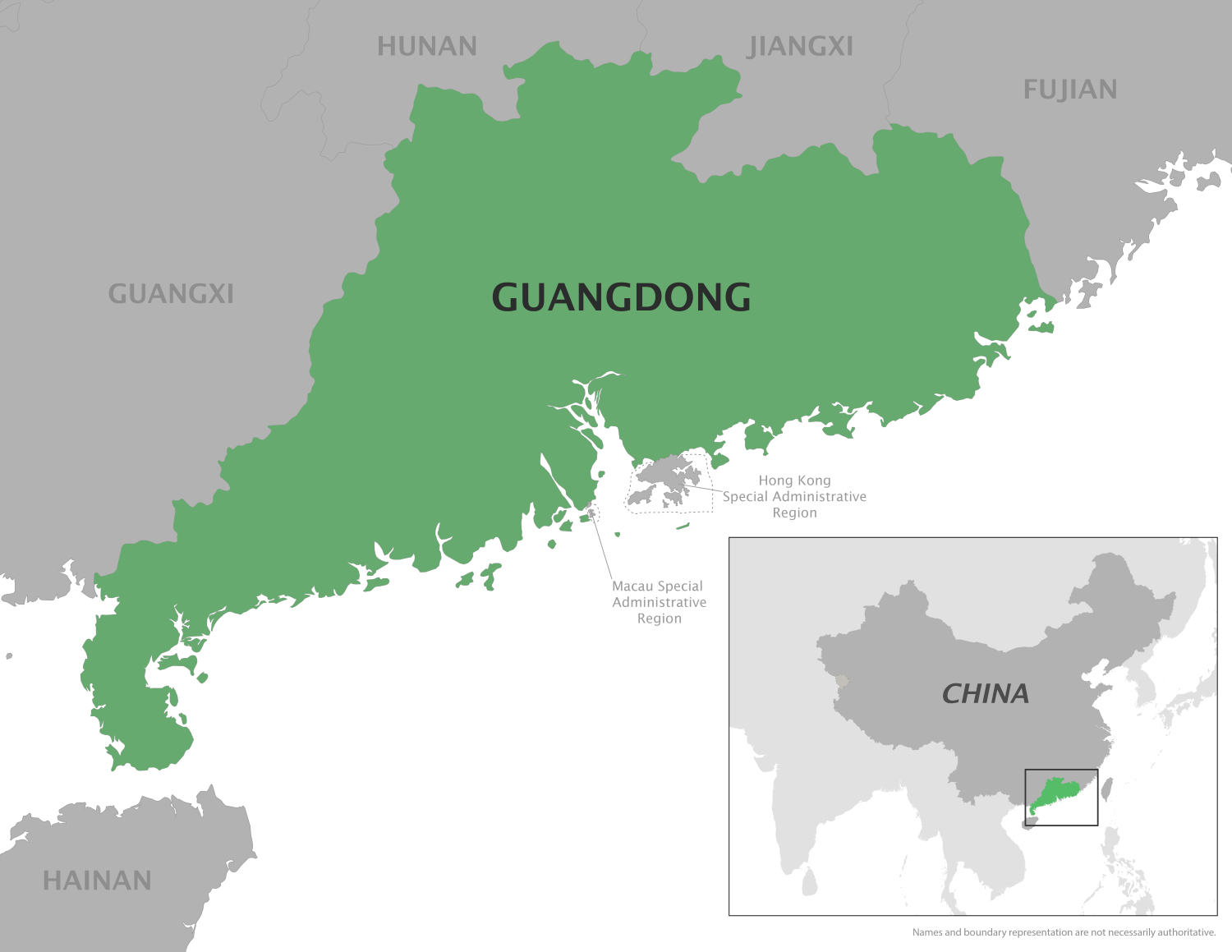
The U.S. Centers for Disease Control and Prevention (CDC) has reissued a Level 2 travel notice, advising travelers to practice enhanced precautions due to the ongoing Chikungunya fever outbreak in Guangdong Province, Republic of China.
Since July 2025, this mosquito-transmitted virus has been challenging China's public health systems on the mainland,
And in Hong Kong, where over 10 local Chikungunya cases have been reported, the popular hiking area, Tsing Yi Nature Trails, was recently closed.
These outbreaks highlight the need to improve surveillance of viral mutations.
According to China CDC Weekly (7(49): 1528-1532), the circulating strain of the Chikungunya virus in Foshan, Guangdong, belongs to the East/Central/South African genotype, not the Indian Ocean Lineage. However, the specific mutations in the viral genome remained unclear.
China's CDC wrote that these mutations are known to enhance viral replication and virus transmission efficiency in Aedes albopictus mosquitoes.
On December 17, 2025, the U.S. CDC stated that we can protect ourselves from this disease by preventing mosquito bites and that vaccination is recommended for travelers visiting an area with a chikungunya outbreak.
The CDC also advises pregnant women to reconsider travel to these affected areas.
If you do visit these areas of China, seek medical care immediately if you develop fever, joint pain, headache, muscle pain, joint swelling, or rash during or after travel.
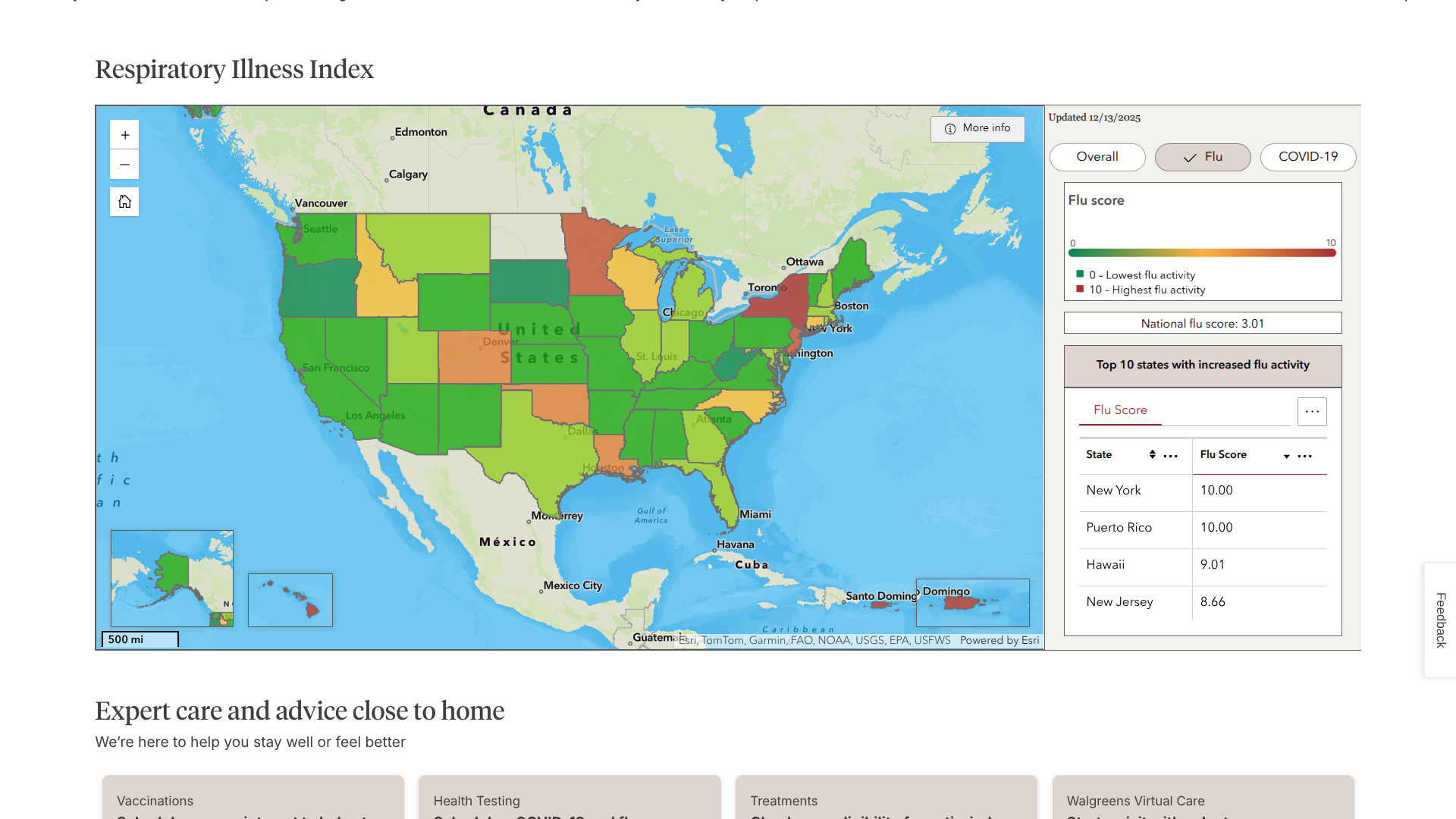
Based on updated data published by the Walgreens Respiratory Illness Index, vacationers visiting a few favorite locations this winter should get their annual flu shot before traveling.
The Index recently ranked New York, Puerto Rico, and Hawaii at the top of its list of influenza-related activity.
By analyzing prescription data, the Index helps people see where influenza activity is high and guides symptoms and when to seek care.
In a press release on December 3, 2025, Rachel Toothman, Walgreens pharmacist and director of pharmacy and retail for the Detroit, Michigan, and Cleveland, Ohio areas, shared quick tips to help people protect their health during travel.
"Keep your hands clean by washing often and using plenty of sanitizer," Toothman says. "Hand hygiene is the first line of defense."
"You may consider immune boosters before your trip, like Vitamin C."
"The No. 1 way to protect yourself and others is to get vaccinated for the respiratory diseases most common this time of year," Toothman added.
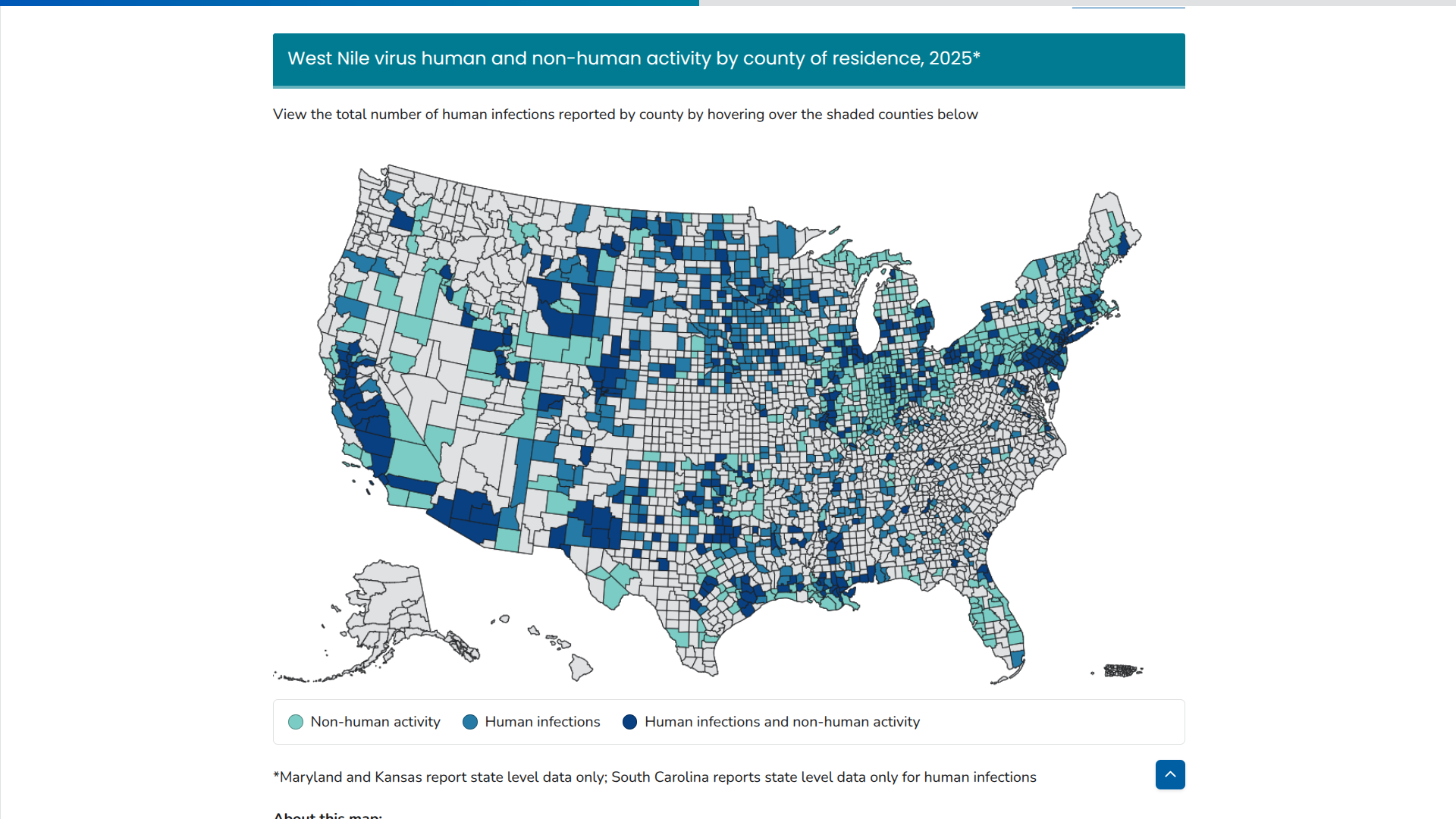
The leading mosquito-borne disease in the United States is not Chikungunya or Dengue; it's the seldom-discussed West Nile Virus (WNV).
Now found in 45 states, WNV causes more illnesses and deaths than any other mosquito-borne disease in the continental U.S., says the U.S. CDC.
As of December 17, 2025, the CDC reported over 2,000 individuals infected with WNV this year. Colorado is an unfortunate leader with 284 cases. Texas has reported 4 WNV-related deaths.
And 1,404 WNV-related Neuroinvasive disease cases in 2025
AMA infectious diseases director Erica Kaufman West, MD, recently addressed the ongoing risks of WNV in the USA. =
A study published by the JAMA Network Open (Vol. 8, No. 12) concluded that older people with a history of chronic kidney disease or conditions affecting blood flow to the brain have about double the risk for developing neuroinvasive disease that can lead to paralysis and death following WNV infection.
"In 2025, there's been a substantial increase in West Nile virus activity with 41% more severe-disease cases and 32% more deaths than what is typically seen," Dr. Kaufman West said in a press release on December 5, 2025.
Physicians don't always have WNV on their radar in patients at high risk for infectious disease—but they should, the AMA added.
And in Europe, 14 countries have reported a total of 1,112 locally acquired human cases.
The countries with the highest number of WNV cases include Italy (779 cases, 72 deaths), Greece (96), and France (62), followed by Serbia (62), Romania (49), and Spain (36). Other countries reporting cases are Hungary (14), Croatia (4), Albania (3), and Germany (2), along with North Macedonia (2), Bulgaria, Kosovo, and Türkiye.
Unfortunately, there have been 97 reported deaths due to the virus in Europe in 2025.
According to the European CDC, these figures are lower than those seen in 2018, 2022, and 2024, years when virus circulation was particularly intense. Italy's data in 2025 represents the highest number of human WNV cases ever reported in a single year.
Both the CDC and ECDC say there is no licensed WNV vaccine for humans yet.
However, several candidates are in clinical trials, focusing on technologies such as live-attenuated or DNA-based vaccines. Therefore, personal protection is the primary human prevention tactic.
While human vaccines are pending, effective equine vaccines are available and widely used, alongside mosquito control.
Since the Zika virus was first detected in the Americas in May 2015, 52 countries and territories have confirmed locally transmitted cases.
While mosquito-transmitted diseases like Zika are detected mainly through passive surveillance systems that identify infections when people seek medical care, countries such as the Republic of El Salvador have taken proactive diagnostic measures.
The country's Ministry of Health monitors for three arboviruses, Chikungunya, Dengue, and Zika, when a person is tested at a facility. And adapted the "It's in your hands" campaigns to include Zika.
As a result, over the last three years, the El Salvador has reported a decreasing number of Zika cases to the Pan American Health Organization (PAHO).
This Central American country reported 106 Zika cases in 2023, 92 in 2024, and 44 as of December 17, 2025.
This data contrasts with a much higher number of Chikungunya and Dengue cases.
Currently, the U.S. CDC does not recommend pre-trip vaccinations for these diseases, but it did include El Salvador in recent Dengue and Measles Travel Health Notices.
And in 2024, the local U.S. Embassy issued a Health Alert due to an increase in Dengue cases.
El Salvador is experiencing a significant tourism spike in 2025, with over 3 million visitors so far. When visiting El Salvador, the Embassy recommends enrolling in the Smart Traveler Enrollment Program to receive digital alerts and make it easier to locate you in an emergency.

Over the last few months of 2025, the Florida Department of Health (FDH) has reported an uptick in travel-related Chikungunya fever cases. During week #50, 46 additional Chikungunya cases were confirmed.
As of December 13, 2025, FDH reported two hundred six travel-associated chikungunya cases in Florida this year.
Florida counties confirming cases include, but are not limited to, Broward (20), Hillsborough (20), and Miami-Dade (127).
FDH says these people recently arrived from Bangladesh, Bolivia, Brazil, and Cuba (194).
Cuba's 2025 Chikungunya outbreak began in western provinces like Matanzas and has reached the capital, Havana.
FDH says Chikungunya is an infection caused by a virus transmitted through the bite of an infected mosquito, similar to Dengue. Initially reported in 2006, Chikungunya has become a locally acquired and travel-related health concern in most years in Florida.
Over the last two years, Chikungunya vaccines have been approved and are available in Florida at various clinics.
As of December 17, 2025, the U.S. CDC recommends pre-departure vaccination before visiting a Chikungunya outbreak zone, such as Cube.
The Pan American Health Organization (PAHO) is urging countries in the Americas to strengthen immunization activities as measles cases continue to rise in the region.
So far in 2025, over 10,000 confirmed measles cases have been reported across ten countries, representing a 34-fold increase compared to the same period in 2024.
On December 15, 2025, the PAHO stated in a press release that these measles outbreaks are linked primarily to low vaccination coverage, with 71% of cases occurring in unvaccinated individuals.
Countries with the highest case numbers include Canada (4,548), Mexico (3,911), and the United States (1,356). Other countries reporting confirmed cases are Bolivia, Argentina, Belize, Brazil, Paraguay, Peru, and Costa Rica.
Mexico leads measles-related fatalities with 14 this year.
"Measles is preventable with two doses of a vaccine, which is proven to be very safe and effective. To stop these outbreaks, countries must urgently strengthen routine immunization and conduct targeted vaccination campaigns in high-risk communities," said Dr. Daniel Salas, Executive Manager of the Special Program for Comprehensive Immunization at PAHO, in a press release.
As of mid-December, Canada lost its measles elimination status after three decades, and Mexico is also at risk of losing its measles-free status in the months ahead.
To alert international travelers to their health risk, the U.S. CDC published a Level 1 Travel Health Notice stating that all international travelers should be fully vaccinated against measles with the measles-mumps-rubella (MMR) vaccine.
MMR vaccination services are generally offered throughout the United States at travel clinics and pharmacies.
For a few decades, the Bacillus Calmette-Guérin (BCG) vaccine has been a standard, effective, low-cost immunotherapy for non-muscle-invasive bladder cancer (NMIBC).
However, the 100-year-old BCG vaccine alone did not help all patients with bladder cancer recover.
Recently, the U.S. Food and Drug Administration approved ANKTIVA®, with BCG, for the treatment of patients with BCG-unresponsive NMIBC with carcinoma in situ (CIS), with or without papillary tumors.
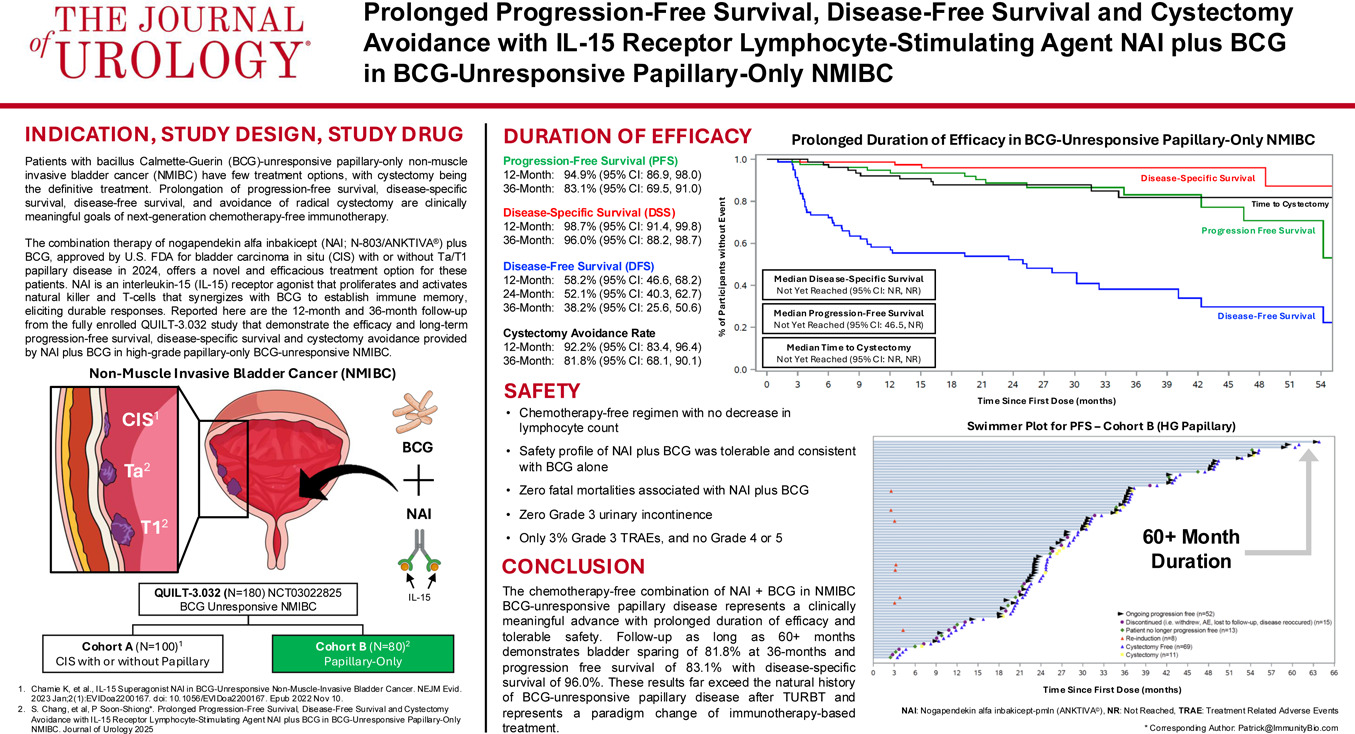
An Original Research article published in the Journal of Urology, January 1, 2026, edition, demonstrates efficacy at 12 and 36 months, including disease-free survival, disease-specific survival, long-term progression-free survival, and high cystectomy avoidance in patients with BCG-unresponsive high-grade papillary-only NMIBC.
These researchers stated the findings also show tolerable safety consistent with BCG treatment alone, with 3% grade 3 and no grade 4 or 5 treatment-related adverse events.
"Patients with BCG-unresponsive papillary-only non-muscle invasive bladder cancer have few treatment options, with cystectomy being considered the definitive treatment," said lead author Sam S. Chang, M.D., Professor of Urology and Chief Surgical Officer of the Vanderbilt Ingram Cancer Center, in a press release on December 16, 2025.
"Prolongation of progression-free survival, disease-specific free survival, and avoidance of bladder removal are clinically meaningful goals of next-generation chemotherapy-free immunotherapy."
"Our findings provide evidence that ANKTIVA plus BCG would offer a novel and efficacious treatment option for these patients."
ANKTIVA is currently approved in the USA and the United Kingdom, and has a Conditional Marketing Authorization in the European Union, with BCG, for the treatment of patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors.
"The evidence that CIS and papillary disease are clonally linked, combined with the QUILT-3.032 findings showing long-term cystectomy avoidance, sustained avoidance of progression to muscle-invasive disease, and 96% bladder cancer-specific survival at three years, supports the consideration that ANKTIVA plus BCG addresses the unmet need for patients with papillary disease alone who face the prospect of total radical cystectomy following failure of BCG therapy," added Dr. Patrick Soon-Shiong, Founder, Executive Chairman and Global Chief Scientific and Medical Officer of ImmunityBio.