Search API
According to Spanish media, a man residing in the Community of Madrid is the first Mpox clade 1b case of autochthonous (local) infection of the most contagious strain of the sexually transmitted virus.
The Madrid Directorate General of Public Health announced in late September 2025 that the patient is in home isolation and is progressing favorably. His close contacts are being investigated to identify potential secondary cases.
As of early October 2025, all reported cases of this Mpox variant in Europe had been imported, primarily from African countries.
According to the latest epidemiological bulletin from the Carlos III Health Institute, since 2022, Spain has detected a total of 9,027 cases of Mpox, 487 of them this year.
The U.S. CDC states that Mpox is a vaccine-preventable disease, with various vaccines approved for human use and commercailly available at select pharmacies.

The Costa Rican Ministry of Health recently reported a positive case of yellow fever following the results issued by the laboratory of the Costa Rican Institute for Research and Teaching in Nutrition and Health.
This is a 29-year-old American patient who arrived from the Amazon region of Peru on October 8, 2025. According to available information, the patient had not been vaccinated against yellow fever. She is currently hospitalized in a Costa Rican Social Security Fund hospital, where she is receiving specialized medical care.
In a media release on October 12, 2025, the Health Ministry wrote It's worth noting that the patient's partner, who has been vaccinated against yellow fever, tested negative for the disease after undergoing laboratory tests.
The institution also reiterates the importance of all people planning to visit countries endemic for yellow fever, such as Bolivia, Brazil, Ecuador, Guyana, French Guiana, Paraguay, Peru, Venezuela, Suriname, and tropical African countries, getting vaccinated at least 10 days before their trip.
A yellow fever vaccination (YF-VAX or Stamaril) certificate may be required at the airport for those visiting Costa Rica.
In 2017, the Centers for Disease Control and Prevention expanded its yellow fever vaccine recommendations for certain travelers.
To inform Macao residents about the SAR Government's overall prevention and control strategy and specific implementation measures for Chikungunya and dengue fever as early as possible, the Health Bureau held a press conference today to introduce the current epidemic situation.
As of October 11, 2025, Director Luo Yilong stated in a media release that since the first imported case of Chikungunya in Macao at the end of July, Macao has recorded 32 cases, of which 24 were imported and eight were locally infected by mosquitoes carrying the virus.
Considering the situation in neighboring regions and the flow of people, the risk of importing Chikungunya into Macao continues to exist. However, at present, local cases are still sporadic, and no clusters have occurred.
Medical Director Zeng Tanfei and Attending Physician Tian Yakun introduced the treatment of patients with double fever at the Conde S. Januario Hospital. As of October 12, 2025, there were six patients currently receiving hospitalization for double fever at the Conde S. Januario Hospital, four of whom were diagnosed with Chikungunya and two with dengue fever, none of whom had serious complications.
Macao, an autonomous region on the south coast of China, advises that residents with questions can contact the Health Bureau's infectious disease hotline at 28700800.
Furthermore, the outbreak of Chikungunya in Guangdong Province, China, continues.
To enhance disease awareness in China, the first Chikungunya virus detection product was approved in October 2025 for marketing in the country. It can be used for the in vitro diagnosis of Chikungunya cases.
As of October 13, 2025, the U.S. CDC includes this area of China in a Travel Health Notice and recommends vaccination for specific travelers ahead of departure.
UNICEF, the WHO, and Gavi warned in April 2025 that vaccination disparities persist in Bangladesh, raising serious concerns about the long-term health of most children.
Despite the remarkable progress of the country's Expanded Program on Immunization (EPI), children remain under-immunized, and about 1.5% have received no vaccines at all.
According to Reuters reporting, Bangladesh launched a nationwide typhoid vaccination campaign on October 12, 2025, to immunize approximately 50 million children.
The month-long campaign, conducted free of charge under the government's EPI, targets children between nine months and 15 years old.
The U.S. CDC recommends typhoid vaccination for most travelers visiting Bangladesh, particularly those staying with friends or relatives or exploring smaller cities or rural areas.
Additionally, the CDC has highlighted health risks related to Chikungunya, Dengue, and Measles when visiting this Southeast Asia country in 2025.

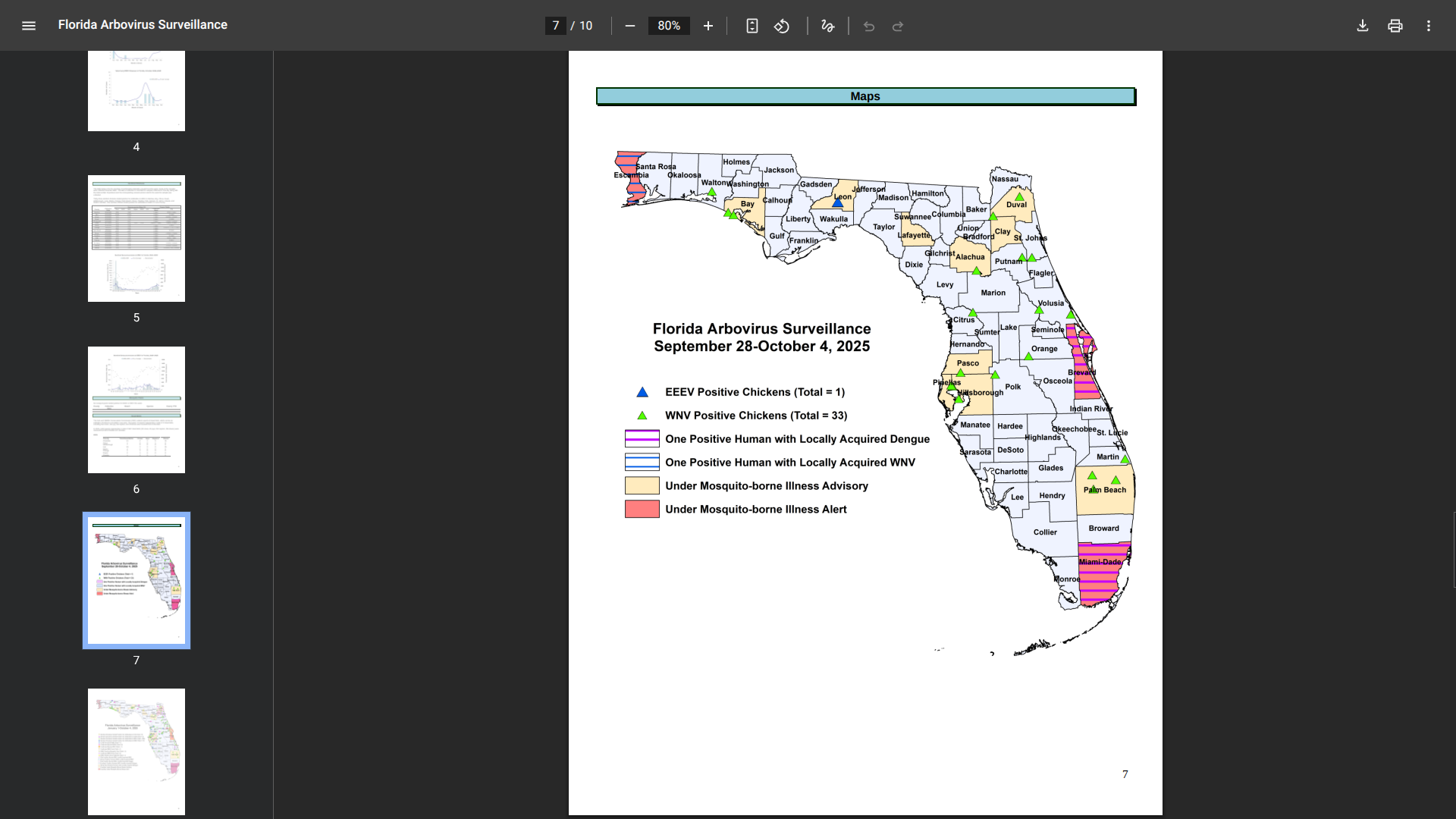
The Florida Department of Health (DOH) recently reported additional mosquito-transmitted Dengue Fever cases, one each in Miami-Dade and Brevard counties along the east coast.
In 2025, 47 cases of locally acquired Dengue have been reported in Brevard (31), Hillsborough, Miami-Dade (14), and Pasco counties.
In 2024, 91 locally acquired Dengue cases were reported from ten Florida counties (Miami-Dade (50). Most dengue cases are serotyped as DENV-3 and DENV-4.
Additionally, fifteen cases of Dengue were reported this week in persons who had traveled internationally. In 2025, 254 travel-associated dengue cases were reported, led by 167 people with Cuba-related visits.
To notify residents and visitors of this health risk, the DOH has announced that Alachua, Bay, Clay, Duval, Hillsborough, Lafayette, Leon, Palm Beach, Pasco, and Pinellas counties are currently under a mosquito-borne illness advisory.
And Brevard, Escambia, and Miami-Dade counties are presently under a mosquito-borne illness alert.
As of October 11, 2025, Dengue vaccines are unavailable in Florida, but are being tested on children in Puerto Rico.
French health authorities in the Occitanie region recently reported a fatal rabies case in a resident located in the southernmost administrative region of metropolitan France.
Rabies is not thought to circulate in either wild or domestic animals in France, although some species of bats can carry a rabies-like virus.
Investigations into this rabies infection, which is believed to be related to travel abroad, are ongoing.
As of October 1, 2025, all veterinarians in the metropolitan area, in conjunction with the departmental population protection directorates, have been informed and made aware of this situation.
The last "endemic" case was a fox in Moselle in 1998. After 2 years without any cases, France became officially free in 2001.
Rabies is a vaccine-preventable disease in France and the United States.
According to the U.S. CDC, bats, not dogs, are the leading source of rabies infections in people in the USA.
The Mexican government recently published a report for Epidemiological Week 39 that included data on the northern spread of the New World Screwworm (NWS).
Historically found in South America, Cuba, Haiti, and the Dominican Republic, parasitic flies carrying the NMW virus, which usually infect cattle, have infected 55 people in Mexico in 2025.
Mexico stated on October 6, 2025, that infestations can be severe and lead to sepsis if untreated, but are not contagious. Flies carrying NWS lay eggs in animal and human wounds, causing larvae to feed on living flesh.
These infestations have been found close the the Mexico-United States border, in Nuevo Leon.
The U.S. CDC says once detected, healthcare providers should remove the NWS larvae from the affected site immediately.
Seperately, Reuters reported the NWS outbreak has rattled the livestock sector in Mexico and the United States since May 2025.
Mexico's agriculture ministry and sanitation agency, Senasica, recently confirmed that ivermectin must be administered 72 hours before the cattle movement, under the supervision of staff from the International Regional Organization for Animal and Plant Health.
Last summer, the U.S. Department of Agriculture implemented extensive plans to prevent the northern spread of NWS.
In October 2025, the U.S. Food and Drug Administration conditionally approved Dectomax-CA1 (doramectin injection) injectable solution for the prevention and treatment of NWS larval infestations, as well as the prevention of NWS reinfestation for 21 days.
Dectomax-CA1 is conditionally approved for use in cattle, not for human use.
The European Center for Disease Prevention and Control (ECDC) has released updated information on the spread of West Nile virus (WNV) activity in Europe. Case numbers reported so far this year are above the average for the past decade.
During 2025, 13 European countries have confirmed 989 locally acquired cases and 63 related fatalities.
Locally acquired cases have been reported by Italy (714), Greece (91), Serbia (60), France (42), Romania (36), Spain (23), Hungary (11), Croatia (4), Albania (3), North Macedonia (2), Bulgaria (1), Kosovo* (1) and Türkiye (1).
The ECDC says Italy is currently experiencing a large outbreak, including 48 fatalities, with a case fatality rate of 6.7%.
The WNV cases have mainly been reported from the Lazio region (Latina, Roma, and Frosinone), with a total of 252 cases, and the Campania region (Napoli, Caserta, Salerno, and Avellino), with a total of 124 cases.
In the United States, WNV cases have been reported in various states, including Kentucky, in 2025.
Currently, WNV vaccines are in development and unavailable in Europe and the USA.
As of October 10, 2025, the U.S. CDC has not issued a Travel Health Notice regarding Europe's WNV outbreak.

Bavarian Nordic A/S recently reported topline results from a phase 2 clinical study of its MVA-BN® (JYNNEOS®) mpox/smallpox vaccine in children 2 to 11 years of age.
Topline results from the study, comprising 451 individuals evaluable for the primary endpoint, showed that the immune response in children (n=227) two weeks after the second vaccination with MVA-BN was non-inferior to the adult group (n=224), with the highest immune responses observed in the youngest subgroup of children aged 2-5 years.
While the safety and immunogenicity generated from this study in adults were comparable to historical data with MVA-BN, the immune response in children was 2.5 times higher than in the adult group, as demonstrated by neutralizing antibody titers.
The vaccine's safety profile is similar to that of adults and has no unexpected signals.
Nina Wressnigg, Head of Clinical Development Science at the Coalition for Epidemic Preparedness Innovations (CEPI), commented in a press release on October 7, 2025, "Mpox has been raging across Africa for over a year and remains a declared continental health emergency."
"Although MVA-BN has been licensed for emergency use in children in the Democratic Republic of the Congo (DRC) - the worst-affected country - many other countries lack this access, causing children to continue to bear the brunt of the suffering, marked by severe illness and possible loss of life.
"These new topline data provide additional positive findings that could expand licensure to children in more countries to control the ongoing outbreak."
Pending final results from the study, Bavarian Nordic plans to submit the data to the European Medicines Agency (EMA) in 2026 to support an extension of the vaccine's approval to include children aged 2 years and older.
The European Commission currently approves MVA-BN for individuals aged 12 years and older.
The findings could also expand the use of the vaccine to children in countries severely affected by the current mpox outbreak, surging in Africa, with cases also reported in other countries around the world.
MVA-BN or Modified Vaccinia Ankara-Bavarian Nordic is the only non-replicating mpox vaccine approved in the U.S., Switzerland, Singapore, Mexico, Canada (IMVAMUNE®), the EU/EAA, and the United Kingdom (IMVANEX®).
In the United States, JYNNEOS is commercailly available at clinics and pharmacies.
Recently, the Chicago Department of Public Health reported 104 cases of Mpox have been confirmed in 2025. This data is more cases than were reported over the same time period in 2023 (40) and 2024 (53) combined.
The study was co-funded by the CEPI and was conducted at sites in the DRC and Uganda.

