Search API
The U.S. FDA confirmed the 188th Meeting of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) will be conducted on December 12, 2024,
The Briefing Document for this digital meeting states the VRBPAC will meet in open session to discuss considerations for Respiratory Syncytial Virus [RSV] vaccine safety in pediatric populations. The FDA wrote, "The observed imbalance in severe/very severe cases of RSV LRTI in the mRNA-1345 and mRNA-1365 vaccine development program among 5-month to <8-month-old recipients of mRNA-1345 (15 μg) and mRNA-1365 (15 μg) has uncertain implications for the ongoing and future pediatric development of other non-live attenuated RSV vaccines."
Moderna's mRNA-1345 is an investigational RSV vaccine with a single mRNA sequence encoding for a stabilized prefusion F glycoprotein. The vaccine uses the same lipid nanoparticles (LNPs) as in the Moderna COVID-19 vaccines. The F glycoprotein is on the virus's surface and is required for infection by helping the virus enter host cells. It exists in two states: prefusion and postfusion. The prefusion conformation is a significant target of potent neutralizing antibodies and is highly conserved across both RSV-A and RSV-B subtypes.
The draft Agenda overview is as follows:
- Clinical and Nonclinical Aspects of RSV Vaccine Safety in Young Children.
- Review of Investigational RSV (mRNA-1345) and RSV/hMPV (mRNA-1365) Vaccines in Infants and Children < 2 Years.
- Imbalance in Severe RSV Cases in a Clinical Trial of an RSV Vaccine in Infants and Young Children.
- Committee Discussion of Considerations for RSV Vaccine Safety in Pediatric Populations.
The FDA welcomes the attendance of the public at its advisory committee meetings and will make every effort to accommodate persons with disabilities. This is the YouTube link.
Advisory committees provide independent expert advice to the FDA on broad scientific topics or specific products to help the agency make sound decisions based on the available science. Advisory committees make non-binding recommendations to the FDA, which generally follows the recommendations but is not legally bound to do so.
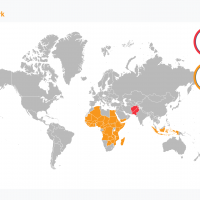
New data published today from the World Health Organization (WHO) reveal an estimated 263 million malaria cases and 597,000 related fatalities worldwide in 2023.
Confirmed on December 11, 2024, this data represents about 11 million more cases in 2023 compared to 2022.
Approximately 95% of the deaths occurred in the WHO African Region.
In the United States, the Centers for Disease Control and Prevention (CDC) reported 1,772 malaria cases as of November 23, 2024 (#47), mainly in international travelers arriving in New York City (232).
“No one should die of malaria, yet the disease continues to disproportionately harm people living in the African region, especially young children and pregnant women,” said Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, in a media release.
“An expanded package of lifesaving tools now offers better protection against the disease, but stepped-up investments and action in high-burden African countries are needed to curb the threat.”
From a prevention perspective, new-generation nets, which provide better protection against malaria than pyrethroid-only nets, are becoming more widely available, supporting efforts to combat mosquito resistance to pyrethroids.
And two malaria vaccines are offered in Africa but not in the U.S.
As of December 2024, 17 countries had introduced malaria vaccines through routine childhood immunization.
"Until a viable malaria vaccine becomes available, travelers visiting malaria risk areas need to take precautions against infection, including antimalarial medication along with application of EPA-approved insect repellents to skin and clothing," commented Jeri Beales, MSN, RN.
"In the U.S., malaria prevention medication is by prescription only, so you'll need to speak with your physician's office or local travel health clinic. Several antimalarial tablet options are available, and the best option depends on where you will be visiting, for how long, and your health history, including medications you already take."
"It's important to remember that all malaria medications need to be taken before, during, and after you visit a high-risk area to be effective," added Beales, who leads Destination Health Clinic, a Boston-area travel health provider specializing in health education and vaccination for international travelers.

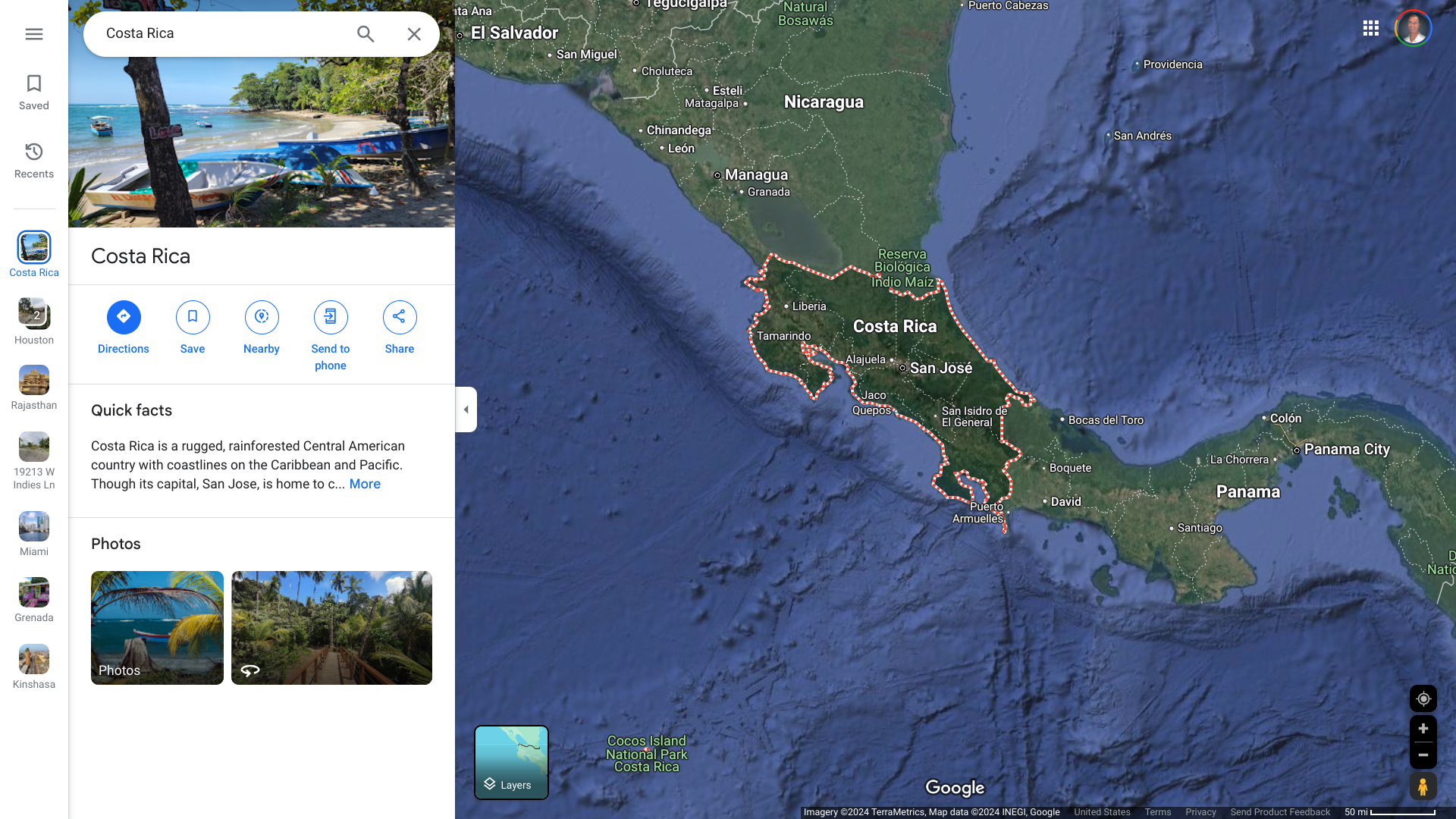
The U.S. Department of State today issued a Level 2: Exercise Increased Caution, Travel Advsiory for the Republic of Costa Rica.
As of December 10, 2024, the State Department confirmed that visitors to Costa Rica should exercise increased caution due to various crimes targeting tourists.
If you travel to Costa Rica, enroll in the Smart Traveler Enrollment Program to receive digital aalerts, making locating you in an emergency easier. Additionally, the U.S. Embassy in San José is located at Calle 98, Via 104, Pavas.
This advisory provides essential information for Costa Rica's expanding tourism industry. Over 1.5 million tourists visited Costa Rica in early 2024, a 14% increase from the same period in 2023.
From a health perspective, mosquito-transmitted Chikungunya, Dengue, Malaria, and Zika viruses have been confirmed and vary by location in 2024. For example, there have been:
Over 29,700 Dengue cases, 40 Chikungunya infections, and 25 Zika cases have been reported this year, primarily in Costa Rica's mountains.
"Zika, Chikungunya, and Dengue Mosquitoes (Aedes aegypti and Aedes albopictus) generally bite from Dawn to Dusk each day," commented Duellyn Pandis, DNP, MS, APRN, FNP-C, Certificate in Travel Health®.
"They may also be active in well-lit areas. They prefer to bite legs, hands, faces, necks, and ears. Protect yourself using appropriate repellants such as DEET and Permethrin for those diseases that are not vaccine-preventable," added Pandis, President & CEO of Passport Health of Tampa Bay.
The U.S. CDC suggests that future visitors to Costa Rica speak with a travel vaccine expert at least one month before traveling abroad about disease protection options. Travel vaccines are available in health clinics and pharmacies in 2024.
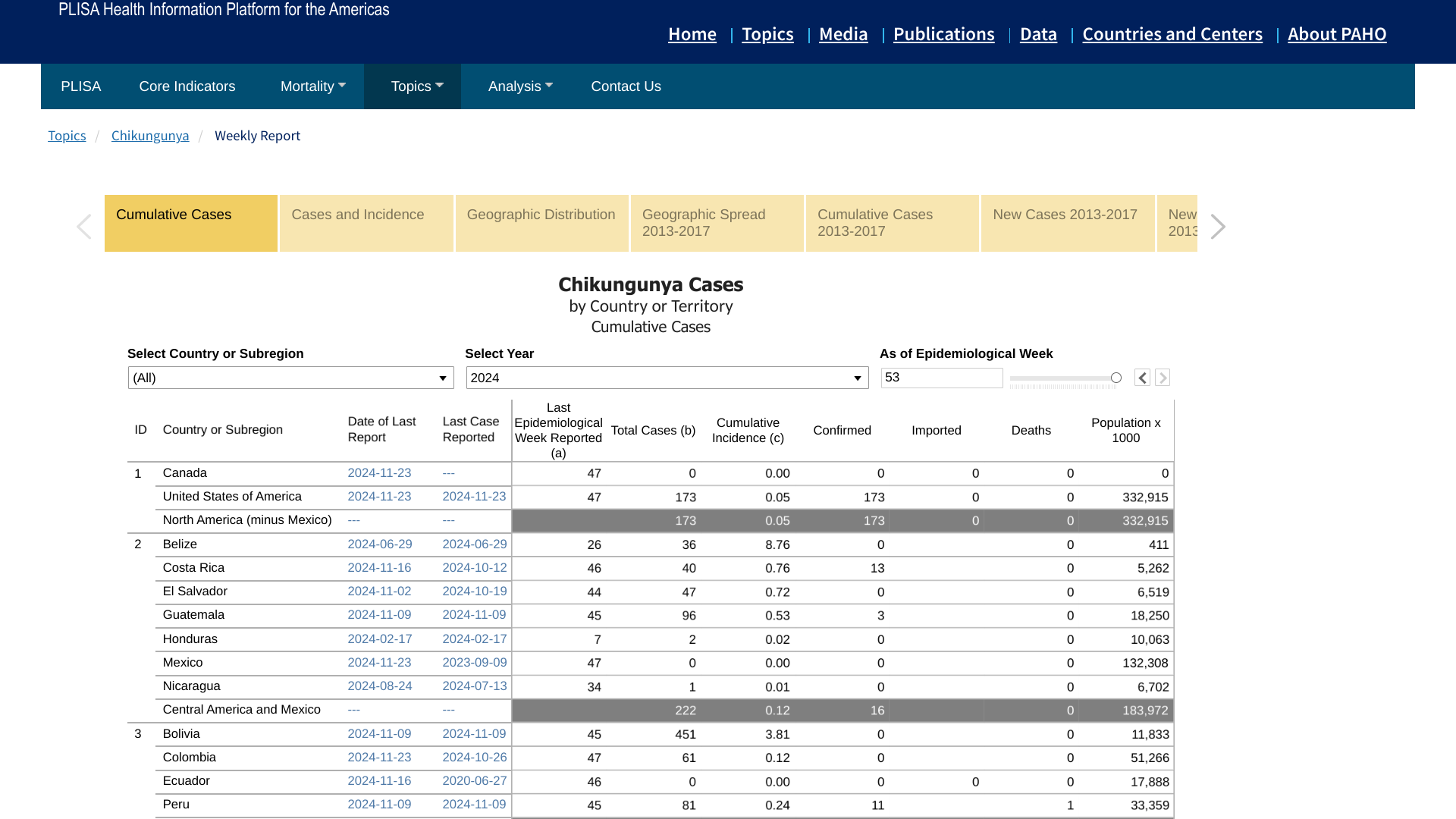
Over the past decade, Chikungunya virus disease has become one of the most neglected arboviral diseases transmitted by infected mosquitoes. During 2024, Chikungunya outbreaks were confirmed throughout the Region of the Americas, infecting over 412,000 people.
While Chikungunya does not often result in death (204 people in 2024), the joint pain associated with the disease may last for months or years and may become a cause of chronic pain and disability.
Unlike previous years, a highly effective U.S. FDA-approved vaccine is commercially available in the United States and various other countries.
To quantify the effectiveness of Valneva SE's IXCHIQ® (VLA1553) monovalent, single-dose vaccine, The Lancet Infectious Diseases, Robert McMahon and colleagues reported in December (Volume 24, Issue 12, p1298-1299) their single-arm, multicentre, phase 3b clinical trial assessing antibody persistence and safety up to 2 years after a single vaccination with the chikungunya virus vaccine.
Valneva announced on December 3, 2024, that among healthy adults still enrolled in the trial, 96% maintained neutralizing antibody titers well above the seroresponse threshold three years after the single-dose vaccination.
The U.S. CDC encourages international travelers to speak with a travel vaccine expert at least one month before visiting Chikungunya-endemic areas in 2024.
The World Health Organization (WHO) today announced the 43rd situation report for the multi-country outbreak of mpox, including countries in the African Region and some countries in the Eastern Mediterranean Region.
As of December 9, 2024, most (>95%) of suspected mpox cases in all African countries except the Democratic Republic of the Congo are tested, so only confirmed cases are reported.
The highlights of this 26-page report are as follows:
- The Emergency Committee under the International Health Regulations was unanimous in expressing the view that the ongoing upsurge of mpox still meets the criteria of a public health emergency of international concern (PHEIC) and that the event continues to constitute a PHEIC and issued revised temporary recommendations to this effect.
- Since the last situation report, two additional countries have confirmed travel-related cases of mpox due to clade Ib MPXV for the first time: Canada and the United States of America. The U.S. case is a male adult in California who reported a recent history of travel to locations in East Africa.
- In late November 2024, the United Kingdom notified WHO of a fifth case of mpox in Leeds due to clade Ib MPXV.
- One country, Angola, has reported mpox cases for the first time.
Regarding preventive vaccines, following the vaccination workshop in early November 2024, countries are submitting updated vaccination plans. As of December 2024, 1,678,000 additional mpox vaccine doses are available for shipment.
In addition to Bavarian Nordic's JYNNEOS® (MVA-BN®) vaccine, the WHO added LC16m8 to the Emergency Use Listing in November 2024. A single dose of the Japan-based vaccine is administered via a multiple-puncture technique using a bifurcated needle.
In the U.S., the JYNNEOS vaccine is commercially available at various clinics and pharmacies.

Every year, malaria-infected travelers return to the UK after visiting countries in Africa. Last year, 2,106 malaria cases, with six deaths, were reported in the UK by returning travelers. This data is 26% higher than confirmed in 2022.
With the winter holiday season licking off, UK health officials are preparing for an influx of malaria cases.
To better treat these people, the United Kingdom Health Security Agency (UKHSA) UK Malaria Expert Advisory Group recently published updated Malaria prevention guidelines for travelers from the UK.
This information is also helpful for travelers interested in antimalarial options.
As of December 3, 2024, the UKHSA says these enhanced malaria guidelines are a practical resource for health professionals advising travelers to Africa. Get pre-travel advice as soon as possible, ideally four to six weeks before you travel, although last-minute advice is still important if time is short.
While Heathrow Airport is England's busiest international depot, numerous healthcare providers are in the surrounding cities, ready to offer pre- and post-trip travel vaccine advice.
And for travel insights, malaria maps for the Central African Republic, Guyana, Nicaragua, and Venezuela have also been created, and an enhanced map for South Africa has been produced.
Also, as of December 9, 2024, two malaria vaccines are being offered in Africa, with limited distribution elsewhere.

The Pan American Health Organization (PAHO) is conducting a briefing on the current state of dengue, Oropouche, and avian flu in the Region of the Americas. These Arthropod-borne viruses pose a significant public health threat, with a notable expansion in their geographic spread.
Recently, the Oropouche virus has also expanded in certain countries, such as Cube, and avian flu has been reported in birds, mammals, and humans along the northern flyway.
On December 10, 2024, at 10:00 a.m. EST, via Zoom with prior registration, the PAHO will provide an update on the situation of these viruses and recommendations for 2025.
Since 2003, the Americas have faced an unprecedented increase in dengue outbreaks in 2024, with over 12.6 million infections and 7,713 related fatalities, marking a record year.
In the United States, the CDC reported that 52 jurisdictions, led by California, Florida, New Jersey, New York, and Puerto Rico, had reported 7,858 dengue cases this year. According to local reporting, dengue may have become endemic in Puerto Rico and Miami, Florida.
As of December 9, 2024, no dengue vaccine is available in the U.S.