Search API
Despite decades of clinical research, Herpes Simplex Virus (HSV) remains among the most prevalent infectious pathogens, impacting millions annually. While herpes vaccine candidates continue to progress in clinical trials, none have been approved.
However, an innovative HSV therapy may soon become available.
Theralase® Technologies Inc. announced today that the previous University of Manitoba research has been validated, proving that Ruvidar® is safe and effective in the inactivation of Herpes Simplex Virus, Type 1 (HSV-1), in an animal model.
In the latest Theralase® research, announced on February 10, 2025, Balb/C mice were infected with the human HSV-1 virus. On day 6 post-infection, 20 uL of a 1% Ruvidar® solution was applied topically over the area of well-developed lesions once daily for four days.
Four days of Ruvidar treatment resulted in complete healing of the HSV-1 cutaneous lesions.
In a press release, the Company stated that these 'results support the safety and efficacy of topically applied non-light activated Ruvidar® against cutaneous HSV-1 lesions in a mouse model.'
Kevin Coombs, B.A., M.A., Ph.D., professor of medical microbiology and infectious diseases at the Max Rady College of Medicine, University of Manitoba (retired), stated, "I am delighted that Theralase® researchers were able to successfully translate my team's cellular inactivation of HSV into a safe and effective therapy in an animal model."
"Their research may prove to be instrumental in developing a clinical program that will have real-world impacts on the lives of billions of people infected with this prolific disease."
Effective U.S. FDA-approved anti-herpetic drugs available in 2025 include acyclovir and later-generation derivatives (penciclovir, valacyclovir, famciclovir, and ganciclovir), which inhibit viral DNA synthesis.
Independent research by the University of Manitoba verifies that Ruvidar is more effective than acyclovir in inactivating HSV after infection.
According to the Company's press release, Ruvidar inhibited HSV-1 replication at significantly lower concentrations.
The effects of Ruvidar versus acyclovir on HSV-1 yields when added 24 hours post-infection (hpi). Vero cells were infected with HSV-1 at a Multiplicity of infection ~ 1.5, incubated for 24 hours, and then treated at 24 hpi with indicated drug concentrations for an additional 44 hours.
Virus yields were then determined, and reductions in virus yields were compared to non-treated controls.
Roger DuMoulin-White, B.Sc., P.Eng, Pro.Dir., President and Chief Executive Officer, Theralase, stated, "Based on the success of Theralase®'s latest research, Theralase® plans to develop a vaccine and therapeutic for the prevention and treatment of HSV, with clinical development to commence thereafter."
This announcement was based on an animal model study, indicating the product is not commercially available.

While the U.S. Centers for Disease Control and Prevention (CDC) has issued measles outbreak advisories for 59 counties, it may soon issue one for western Texas, which has reported 25 measles cases in 2025.
On February 11, 2025, the Texas Department of State Health Services (DSHS) reported that the ongoing measles outbreak in Gaines County has exceeded previous records.
The local health department, South Plains Public Health District (SPPHD), has confirmed 24 measles cases with symptom onset within the last two weeks. Nine of the patients have been hospitalized.
All of the cases are unvaccinated and residents of Gaines County.
DSHS wrote, 'Due to the highly contagious nature of this disease, additional cases are likely to occur in Gaines County and the surrounding communities.
DSHS is working with SPPHD and Lubbock Public Health to investigate this measles outbreak.
Additionally, in early February, Lynn County, which is located south of Lubbock and east of South Plains, confirmed one measles case.
Previously, Harris County, located in eastern Texas, reported two measles cases in 2025.
There is no suspected link between the west Texas outbreak and the Harris County cases.
Elsewhere in the U.S., Alaska, Georgia, New York City, and Rhode Island have reported measles cases in 2025.
In 2024, the CDC reported 284 measles cases in 32 jurisdictions.
DSHS and the CDC's Advisory Committee on Immunization Practices recommend that children receive one dose of the MMR vaccine at 12 to 15 months of age and another at 4 to 6 years of age. Each dose lowers the risk of infection and the severity of illness if they are infected.
Children too young to be vaccinated are more likely to have severe complications if they contract the measles virus.
Measles vaccines will generally be offered at community pharmacies in 2025.
Since the clade II mpox outbreak began about three years ago, the U.S. Centers for Disease Control and Prevention (CDC) has issued Travel Health Advisories based on the type of virus.
Historically, clade I have been associated with a higher percentage of people with mpox developing severe illness or dying, compared to clade II.
On February 10, 2025, the CDC reissued a Level 2 Practice Enhanced Precautions advisory regarding the clade I mpox outbreaks in eight Central and Eastern African countries.
The CDC wrote, 'There is an ongoing person-to-person transmission of mpox in Burundi, Central African Republic, Democratic Republic of the Congo, Kenya, the Republic of the Congo, Rwanda, Uganda, and Zambia.'
In the United States, the New Hampshire Department of Health and Human Services reported the third clade 1b case in the past four months. This mpox patient recently traveled to Eastern Africa.
During these mpox outbreaks, person-to-person transmission has occurred through various means, including sexual contact, day-to-day household contact, and within the healthcare setting. Transmission has also occurred from contact with certain live or dead wild animals.
Mpox is a disease caused by infection with the Monkeypox virus. Symptoms often include fever, rash, headache, muscle aches, and swollen lymph nodes, although fever is not always present.
The CDC says If you are sick and could have mpox, follow isolation and infection control measures at home and during travel.
Mpox vaccination is recommended by the CDC for certain people visiting at-risk areas.
In the U.S. and many countries, mpox vaccines (JYNNEOS®, MVA-BN®) are commercially available in 2025.
Throughout the Region of the Americas, the Federative Republic of Brazi has been the unfortunate leader in the multi-year Dengue fever outbreak.
In Brazil, the Municipal Health Department of São Paulo reported the most Dengue cases in 2024, about 2.1 million.
To reduce the number of pediatric Dengue cases in 2025, the Municipal Health Department of the capital advised parents and guardians on February 7, 2025, to take children aged 10 to 14 for Dengue vaccination, which occurs at Basic Health Units (UBSs).
UBSs also actively searched the territories in 2024 and 2025 to ensure that Dengue immunization reached this population. The government has estimated 600,000 children in Sao Paulo, and 259,000 first doses (38%) and 134,000 second doses (20%) have been administered to date.
As of February 12, 2025, Takeda's QDENGA® two-dose vaccine is available in Brazil and the Americas but not in the United States.

France is one of the most popular tourist destinations, reaching about 100 million guests annually. Its overseas department, La Réunion, welcomes about 500,000 vacationers to its beautiful mountains and beaches.
However, due to the increase in the number of Chikungunya virus cases and its continued infections, and on the proposal of the Director General of the ARS Gérard COTELLON, Patrice LATRON, France's Prefect of La Réunion, has triggered level 3 of the ORSEC "arboviruses" system, which corresponds to a low-intensity epidemic.
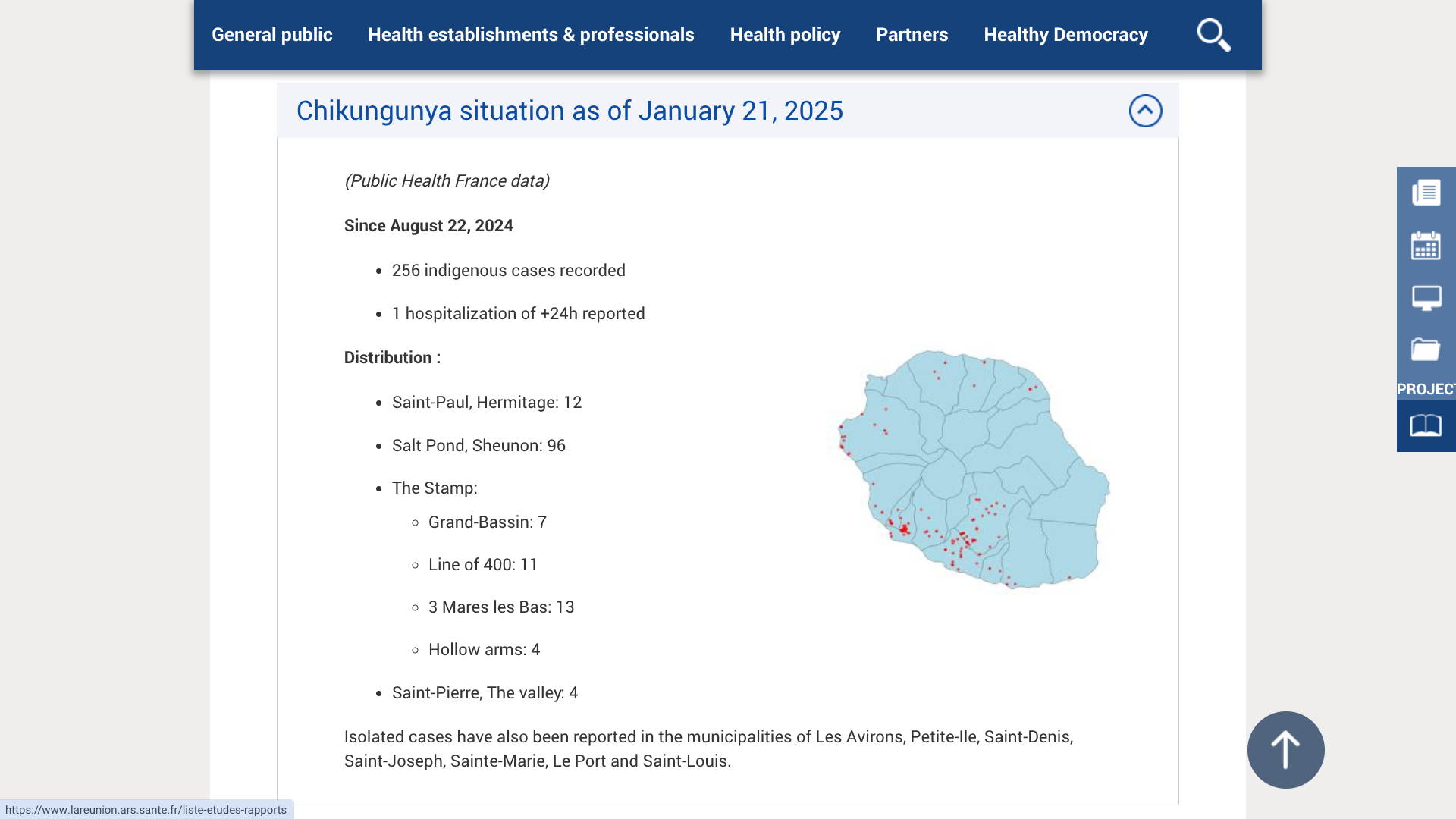
As of February 11, 2025, and since August 23, 2024, ARS Reunion has confirmed 783 indigenous cases, including 671 since the start of 2025.
The municipalities of Étang-Salé and Le Tampon still have the highest number of Chikungunya cases.
'As soon as a case of Chikungunya is reported, the ARS intervenes in the affected area, without waiting for confirmation of the case by the medical biology laboratory, to reduce the risk of spreading the virus.'
The last major Chikungunya outbreak in La Réunion was from 2005 to 2006.
The ongoing outbreak in La Réunion is caused by Ae. albopictus, the primary vector, due to the adaptation of the ECSA CHIKV genotype.
While the U.S. CDC has yet to highlight La Reunion's Chikungunya outbreak as a travel risk, it says international travelers should arrange an appointment with a travel vaccine specialist at least four to six weeks before departing for France. An appointment with a travel expert provides an opportunity to assess which vaccines are appropriate for your trip abroad in 2025.
When departing for France or La Reunion from the United States, Valneva SE's IXCHIQ® chikungunya vaccine is commercially offered by various travel clinics and pharmacies. And in 2025, this innovative vaccine can be found throughout Europe.
The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) recently published a draft agenda for its meeting scheduled for February 26-28, 2025.
This ACIP meeting will be hosted at the CDC in Atlanta, GA, is open to the public, and will be broadcast digitally on YouTube.
On Wednesday, February 26, Dr. Keipp Talbot (ACIP Chair) will welcome the new ACIP members and lead discussions on Meningococcal, Chikungunya, Influenza, Respiratory Syncytial Virus (RSV) disease, and related vaccines.
The ACIP will vote on specific recommendations, which are then sent to the CDC"s Director for consideration and/or approval.
As of January 24, 2025, Susan Monarez, PhD, became the Acting CDC Director, First Assistant to the Director, and Principal Deputy Director.
On Thursday, the 27th, the committee will continue discussing RSV vaccines and then review Human Papillomavirus, Mpox, Pneumococcal, and Lyme diseases.
Then, on Friday, the 28th, COVID-19 and Cytomegalovirus vaccines will be discussed.
The CDC has already scheduled additional ACIP meetings for June 25-26 and October 22-23, 2025.
The ACIP includes up to 19 voting members responsible for making recommendations. The Secretary of the U.S. Department of Health and Human Services selects these members. ACIP voting members are independent medical and public health experts who do not work for the CDC.
These ACIP meetings are essential as the CDC sets the U.S. adult and childhood immunization schedules based on recommendations from ACIP.
According to the Centers for Disease Control and Prevention's recent update, the gastrointestinal illness outbreak on the Royal Caribbean International ship Radiance of the Seas affected 7.4% of its passengers.
Additionally, the crew who reported being ill during the voyage was 8 of 910 (0.9%).
As of February 10, 2025, the writes that it's not clear what caused the outbreak, but said symptoms of infected passengers included vomiting and diarrhea.
The Voyage (20136) was between February 1, 2025, and February 8, 2025.
Per CDC protocol, the Vessel Sanitation Program remotely monitored the onboard situation, including reviewing the ship's outbreak response and sanitation procedures.
This new incident is the 8th in 2025.
The CDC confirmed that 2024 was the worst year for gastrointestinal illness outbreaks (18) on cruise ships in over a decade. Norovirus was the most common cause.
'Norovirus is often a cause of GI illness outbreaks on cruise ships, but we don't always know the cause of the outbreak when we begin an investigation.' writes the CDC.
The CDC says there are no U.S. FDA-approved norovirus vaccines available in 2025.