Search API
GSK plc today announced that the European Commission (EC) had approved a single-vial, fully liquid presentation of Menveo (Meningococcal Group A, C, W-135, and Y conjugate vaccine, MenACWY vaccine) to help protect against invasive meningococcal disease (IMD) caused by bacterial serogroups A, C, W, and Y.
Philip Dormitzer, GSK Head of Global Vaccines Research & Development, commented in a press release on November 27, 2024, “As a leader in meningococcal vaccines, GSK is dedicated to finding innovative solutions that simplify immunization and support vaccine uptake. We remain committed to safeguarding individuals from bacterial meningitis and will persist in our efforts to prevent this devastating disease among at-risk populations in the European Union.”
IMD is an unpredictable, serious illness that can cause life-threatening complications. Despite treatment, among those who contract IMD, up to one in six will die, sometimes in as little as 24 hours.
One in five survivors may suffer long-term consequences such as neurological damage6, amputations, hearing loss, and nervous system problems.
Although anyone can get IMD, babies, young children, and those who are in their late teens and early adulthood are amongst the groups at higher risk.
This single-vial presentation of Menveo is now licensed for active immunization of children from 2 to adolescents and adults. It offers healthcare providers an option that does not require reconstitution before use. More than 82 million doses of this vaccine have been distributed worldwide since 2010.

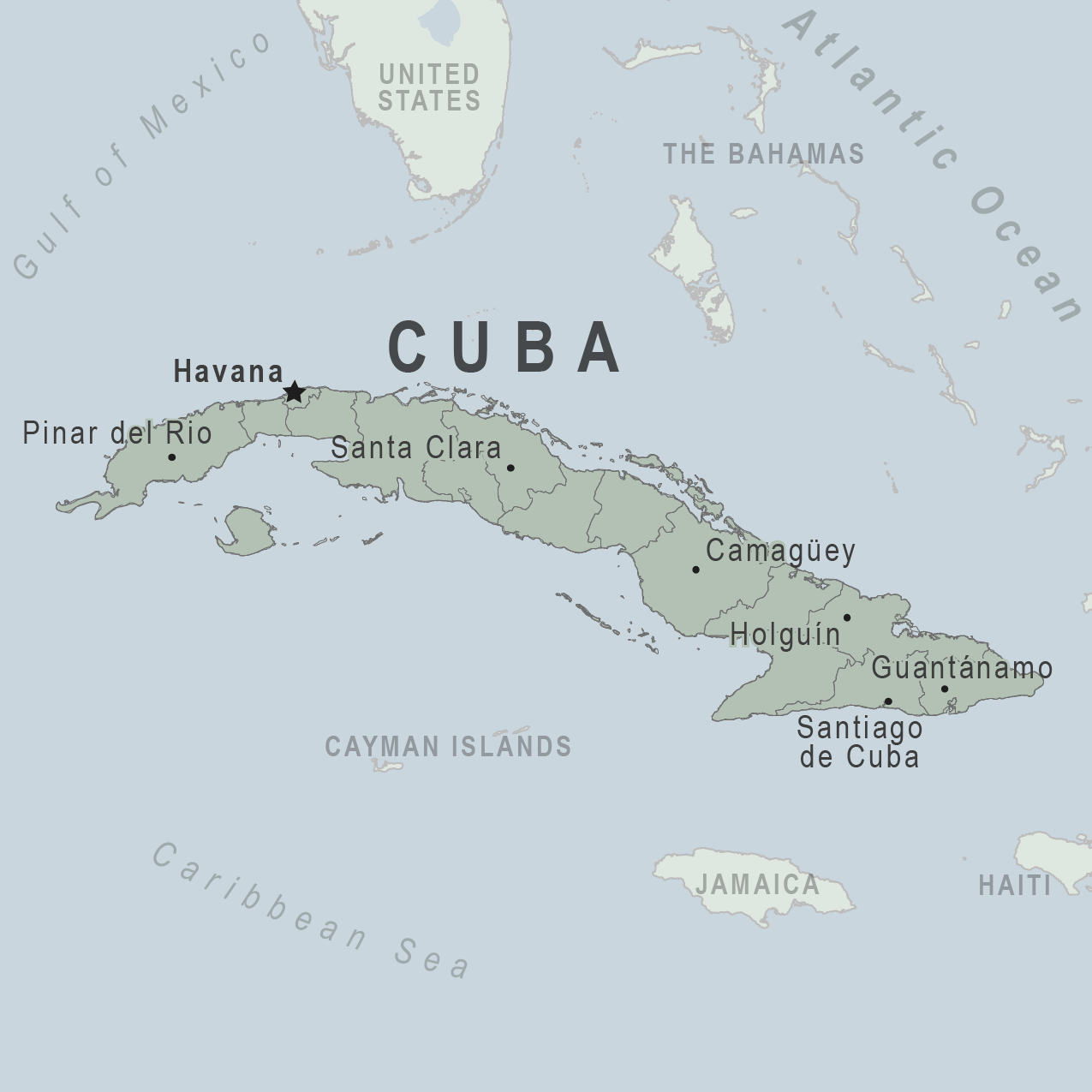
The U.S. Department of State announced on November 21, 2024, that the Department ended the voluntary departure of non-emergency U.S. direct hire employees and their eligible family members due to restoring power to Havana and other clean-up and restoration efforts from Hurricane Rafael.
However, travel by U.S. Embassy employees outside Havana still requires a special notification process, which may affect the Embassy’s ability to provide emergency assistance to U.S. citizens when visiting Cuba.
The Embassy suggests that visitors enroll in the Smart Traveler Enrollment Program to receive alerts and make locating you in an emergency easier. About 200,000 people from around the world visit Cuba monthly.
Seperately the U.S. CDC says to be aware of current health issues in Cuba and vaccination options.
Currently, there is an outbreak of Oropouche in Cuba, with about 90 cases reported in Florida by visitors from Cuba.
Chikungunya, dengue, and zika viruses are year-round risks in many parts of the Americas, including Cuba.
With over 100 countries reporting chikungunya virus outbreaks this year, more international travelers may soon have access to an essential vaccine.
Valneva SE today announced that it has submitted a label extension application to the U.S. Food and Drug Administration (FDA) to potentially extend the use of the only chikungunya vaccine (IXCHIQ®) currently approved in adults and adolescents aged 12 to 17 years.
The application also includes adding the two-year antibody persistence data to the product label, a key differentiator for IXCHIQ®.
This FDA application follows the submission of label extension applications to the European Medicines Agency and Health Canada two months ago. Both Canada and the EMA have already approved IXCHIQ.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release on November 26, 2024, “Given the substantial risk that chikungunya presents to individuals residing in or traveling to endemic regions, it’s imperative to ensure the vaccine is available to all age groups. This broader accessibility would certainly help provide protection and mitigate the burden of this debilitating illness which is currently spreading in areas that were previously unaffected."
"The long-term durability of the immune response from a single shot is also extremely important, especially for endemic countries where access to immunization can be difficult.”
As of November 22, 2024, the Pan American Health Organization reported over 412,089 chikungunya cases and 204 related deaths in the Americas this year.
In the U.S., travel-related chikungunya cases have increased by about 16% in 2024.
The Lancet Infectious Diseases recently published an article showing that IXCHIQ was well tolerated in adolescents 28 days after a single injection, regardless of previous CHIKV infection.
In addition to the adolescent data, the U.S. and Canadian label extension applications included IXCHIQ®’s long-term antibody persistence data, which showed that the vaccine’s immune response was sustained by 97% of participants after 24 months and was equally durable in younger and older adults.
IXCHIQ® was launched in the U.S. at the beginning of March 2024, with launches in France and Canada underway.
In the U.S., IXCHIQ is available at travel clinics and pharmacies.
Valneva expects a marketing authorization in Brazil before the end of 2024 and expanded its partnership with The Coalition for Epidemic Preparedness Innovations (CEPI) earlier this year to support broader access to the vaccine in Low and Middle-Income Countries, post-marketing trials and potential label extensions in children, adolescents and pregnant women.
CEPI will provide Valneva with up to $41.3 million of additional funding over the next five years, with support from the European Union’s Horizon Europe program.
Chikungunya is a mosquito-borne viral disease spread by the bites of infected Aedes mosquitoes, which causes fever, severe joint and muscle pain, headache, nausea, fatigue, and rash. Joint pain is often debilitating and can persist for weeks to years.
A study published by MDPI on October 19, 2024, determined the incidence of post-chikungunya chronic rheumatism and its impact on quality of life and chronic fatigue in adults reached seven years.

The Republic of Mali recently introduced the human papillomavirus (HPV) vaccine into its routine immunization program.
According to Aliou Diallo's report on November 21, 2024, Dr. Ibrahima Diarra, Director of Mali's National Immunisation Centre, highlighted the significance of this development: "A single dose is enough to protect a ten-year-old girl for over ten years against the viruses responsible for 70% of cervical cancers."
Mali has a population of about 22 million and aims to vaccinate more than 320,000 young women annually, potentially reducing cervical cancer cases by nearly 90%.
These vaccinations could, in turn, prevent over 3,600 deaths annually among Malian women.
Thanks to Gavi and co-funding by the government, the HPV vaccine is free for young Malian women. The Sahel stretches across the southernmost latitudes of North Africa between the Atlantic Ocean and the Red Sea.
In 2023, 37 countries were implementing the single-dose HPV schedule.

The Texas Department of State Health Services (DSHS) announced today that the first locally acquired dengue virus case in Texas was reported in Cameron County, the southernmost county.
As of November 25, 2024, 106 travel-associated dengue cases, including one death, have been reported in Travis (Austin) (14), Dallas (13), and twenty other Texas counties.
This is the highest annual case count that DSHS has reported since 2002.
Last year, Texas reported 79 travel-related dengue cases and one locally acquired case in Val Verde County.
Most dengue outbreaks in the U.S. have been linked to travelers visiting endemic areas, including Mexico. Located south of the Rio Grande River, Mexico has reported over 480,000 dengue cases and 262 related deaths in 2024.
From a prevention perspective, no dengue vaccines are currently unavailable in the U.S.

The Public Health Agency of Canada (PHAC) recently confirmed the first case of clade I mpox in Canada.
On November 22, 2024, PHAC announced this travel-related case is associated with an ongoing outbreak of clade I mpox in Africa. The individual sought medical care for mpox symptoms in Manitoba, Canada, shortly after their return and is currently isolating. A public health investigation, including contact tracing, is ongoing.
PHAC stated in a media release that the risk of clade 1 mpox, a virus infection, remains low for the general population. At this time, vaccination of the general public is not recommended.
However, getting vaccinated is a key prevention strategy for those at high risk of exposure. This recommendation includes clade II mpox, which has spread throughout Canada over the past two years.
The Government of Canada has a sufficient supply of mpox vaccines (JYNNEOS®, MVA-BN®, IMVAMUNE®) to support provincial and territorial programs to prevent and control mpox in Canada.
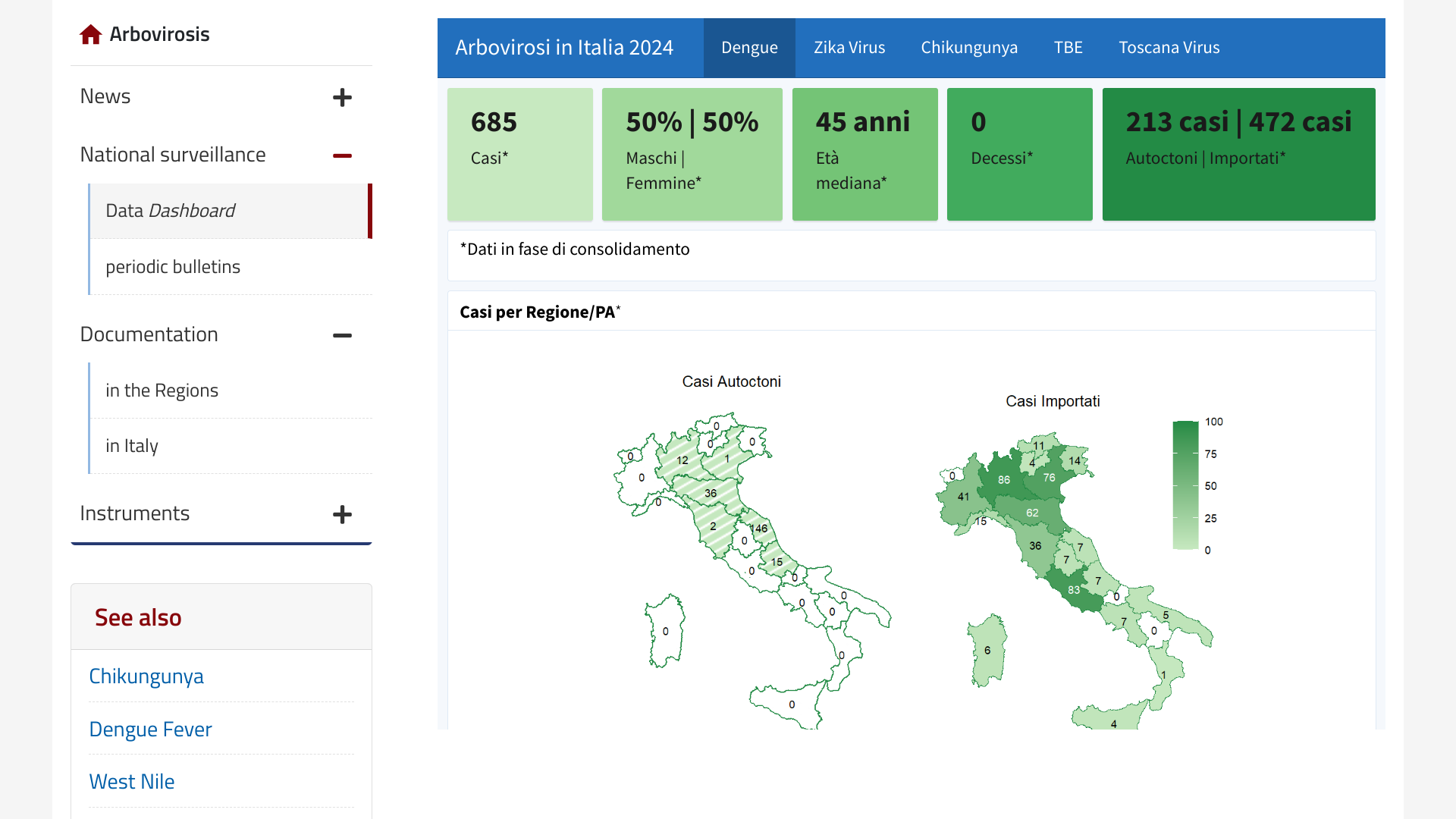
In Europe, dengue outbreaks are primarily associated with infections acquired in endemic countries. Local transmission remains rare, with only sporadic, small-scale outbreaks.
However, according to a Rapid Communication published by Eurosurveillance, Volume 29, Issue 47, on November 21, 2024, Italy's Marche Region experienced a locally-acquired dengue outbreak that peaked in early October 2024.
Fano, a small coastal city, reported autochthonous (local) cases beginning in August. By the end of October, 138 confirmed and 61 probable cases of DENV-2 had been notified.
In total, the Italian National Public Health Authority has reported 213 locally acquired dengue cases in 2024.
Of note, the U.S. CDC's updated Global Dengue Level 1 - Practice Usual Precautions, Travel Health Advisory listed 26 countries, but not Italy.
From a prevention perspective, Takeda's second-generation QDENGA® tetravalent dengue vaccine was approved in Italy in 2023. Unfortunately, it is not offered in the United States in 2024.