Search API
Merck today announced the U.S. Food and Drug Administration (FDA) has accepted the Biologics License Application (BLA) for clesrovimab, the company’s investigational prophylactic long-acting monoclonal antibody designed to protect infants from respiratory syncytial virus (RSV) disease during their first RSV season.
The FDA has set a Prescription Drug User Fee Act date of June 10, 2025.
Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines, Global Clinical Development, Merck Research Laboratories, commented in a press release on December 17, 2024, “We look forward to working alongside the FDA on the review of clesrovimab, which, if approved, would be the first and only single-dose immunization for infants regardless of weight designed to protect them for the duration of their first RSV season.”
If approved, Merck anticipates that clesrovimab shipments will arrive in time for the 2025 RSV season.
Currently, Beyfortus™, an FDA-approved extended half-life monoclonal antibody, is available in the U.S.

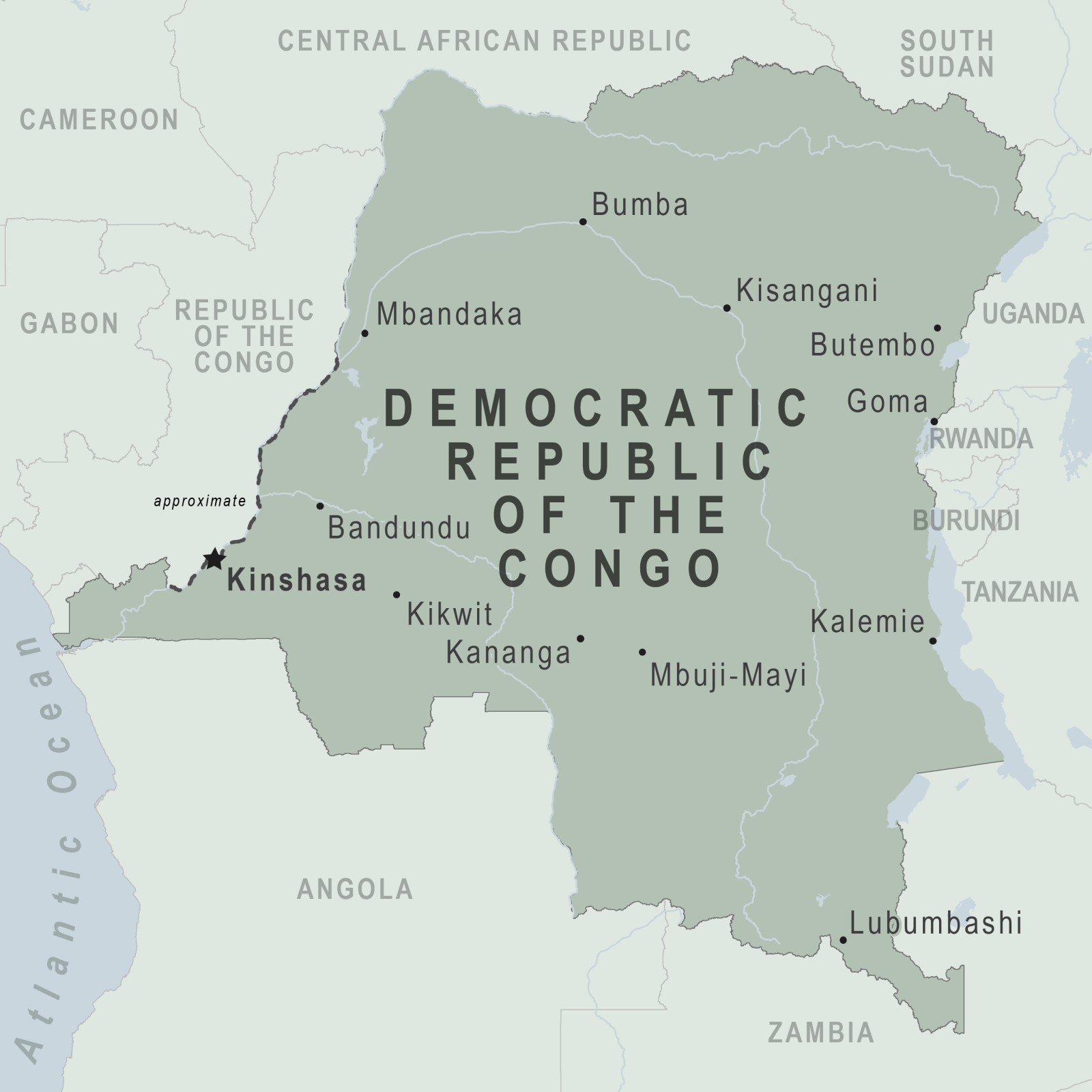
The Democratic Republic of Congo (DRC) Health Ministry informed the media that a previously unidentified disease detected in the Kwango province has been determined to be a form of severe malaria.
The WHO previously reported cases were from nine out of 30 health areas in the Panzi health zone.
"The mystery has finally been solved. It's a case of severe malaria in the form of a respiratory illness," the Health Ministry announced on December 17, 2024. The statement also said that 592 cases had been reported since October 2024, with a fatality rate of 6.2%.
Children aged 0-14 years represent 64.3% of all reported cases.
On December 11, 2024, The Lancet published a Comment that stated, 'the rapid spread of artemisinin partial resistance across east Africa, the Horn of Africa, and southern Africa threatens to undermine malaria control and elimination efforts, potentially increasing deaths.'
The WHO wrote on December 8, 2024, that the overall risk level to the affected communities is assessed as high.
At the regional and global levels, the risk remains low. However, the proximity of the affected area to the border with Angola raises concerns about potential cross-border transmission, and continued monitoring and cross-border coordination will be essential to mitigate this risk.
Two malaria vaccines are currently available in Africa but have yet to be deployed in this area of the DRC.
The U.S. CDC says to visit your healthcare provider at least a month before your trip to the DRC to get vaccines or medicines, as there are current reports of measles, mpox, and polio disease.
The World Health Organization recently reported that measles outbreaks have been reported in 103 countries over the last five years. There were an estimated 10.3 million measles cases in 2023, a 20% increase from 2022.
According to new data from the U.S. Centers for Disease Control and Prevention (CDC), 59 countries outside the Region of the Americas, which includes Canada and the U.S., have reported measles outbreaks this year.
In 2024, the CDC reported 283 measles cases (49 imported) in 32 U.S. jurisdictions, led by Minnesota, with 70 patients.
Additionally, recent bursts of measles cases in Ontario and New Brunswick have increased Canada's total to 160, the highest number in nearly a decade.
On December 16, 2024, the CDC republished its Level 1 - Practice Usual Precautions, Global Measles Outbreak Advisory. The CDC wrote that all international travelers should be fully vaccinated against measles with the measles-mumps-rubella (MMR) vaccine, including an early dose for infants.
Insurance policies generally fund MMR vaccines, available at travel clinics and pharmacies in the U.S.
Furthermore, travelers to at-risk areas should seek medical care if they develop measles symptoms. However, they should alert their healthcare provider before visiting a clinic.

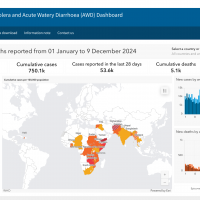
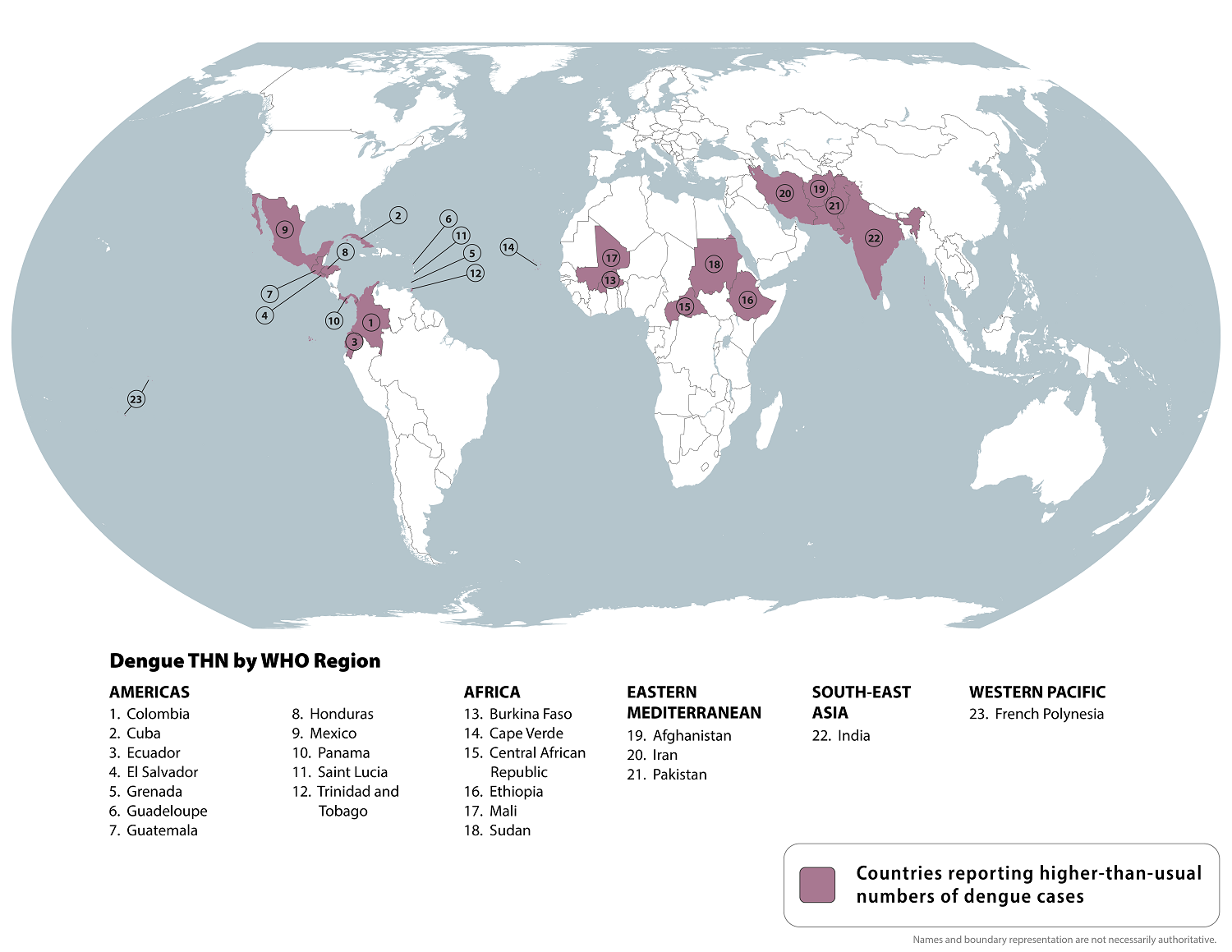
The U.S. Centers for Disease Control and Prevention (CDC) today reissued its Level 1—Practice Usual Precautions, Travel Health Advisory, which highlights Dengue virus outbreaks.
On December 16, 2024, the CDC confirmed that Dengue is mosquito-transmitted and identified 23 countries reporting increased disease cases this year. However, not all countries with Dengue transmission are on this updated CDC list.
Therefore, since Dengue is a year-round risk, international travelers should practice prevention measures for all outbreak areas.
For example, the United States' southern neighbor, Mexico, has reported over 543,000 Dengue cases in 2024.
Within the U.S., the CDC recently confirmed 8,270 travel-related and locally-acquired Dengue cases from 52 jurisdictions, led by Florida (Miami), California (Los Angeles), New York, and Texas.
Although Dengue is a vaccine-preventable disease, no U.S. FDA-approved vaccine was available in late December 2024. However, several vaccine candidates, such as Butantan Institute Butantan-DV, are approaching approval in various countries.
During the peak influenza season in the Northern Hemisphere, health officials strongly encourage most people to get an annual flu shot for protection against respiratory disease.
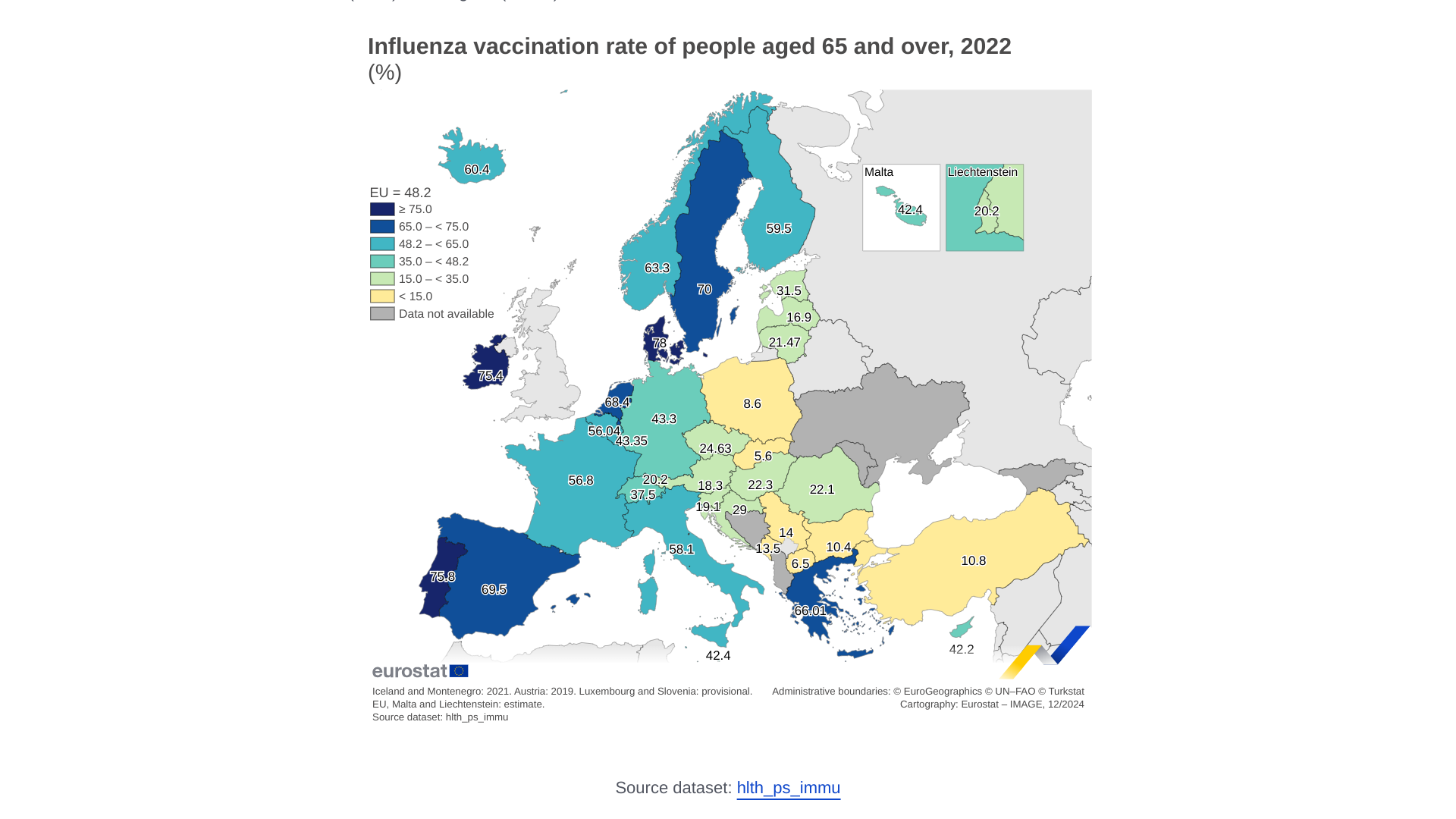
In Europe, influenza vaccination rates have varied over the years.
Eurostat reported on December 16, 2024, that 48.2% of individuals aged 65 or over in the EU were vaccinated against influenza in 2022.
Among EU countries in 2022, Denmark reported the highest vaccination rate for people aged 65 and older at 78.0%, followed by Portugal (75.8%) and Ireland (75.4%). In contrast, Slovakia (5.6%), Poland (8.6%) and Bulgaria (10.4%) had the lowest rates.
The U.S. CDC recommends getting a flu shot before visiting Europe during the 2024-2025 influenza season. Various vaccines are offered at travel clinics and pharmacies in the U.S.
The JAMA Network Open recently published results from an Original Investigation demonstrating respiratory syncytial virus (RSV) vaccine effectiveness in older adults of 90% for RSV–related hospitalization and emergency department visits.
This study provides real-world VE data from the 2023-24 RSV season.
Published on December 13, 2024, these researchers wrote, 'These data (based on data from Kaiser Permanente of Southern California) supports the use of this vaccine (ABRYSVO™) in older adults.'
Pfizer Inc.'s ABRYSVO™ RSVpreF bivalent prefusion F subunit vaccine is a U.S. Food and Drug Administration-approved vaccine.

Stanford University scientists recently reported findings in a mouse study that could lead to a needle-free vaccination approach that eliminates reactions such as fever, swelling, and pain.
Published in the journal Nature on December 11, 2024, Fischbach and colleagues stated the ubiquitous skin colonist Staphylococcus epidermidis elicits a CD8+ T cell response pre-emptively in the absence of an infection.
They wrote that this colonist also induces a potent, durable, and specific antibody response that is conserved in humans and non-human primates.
These specialized proteins can stick to specific biochemical features of invading microbes, often preventing them from getting inside cells or traveling unmolested through the bloodstream to places they should not go.
The initial experiments included dipping a cotton swab into a vial containing S. epidermidis. Rub the swab gently on the head of a regular mouse — no need to shave, rinse, or wash its fur — and put the mouse back in its cage. Draw blood at defined time points over six weeks, asking: Has this mouse’s immune system produced antibodies that bind to S. epidermidis?
The mice’s antibody response to S. epidermidis was “a shocker,” Fischbach said.
“Those antibodies’ levels increased slowly, then some more — and then even more.” At six weeks, they’d reached a higher concentration than expected from a regular vaccination — and they stayed at those levels.
“It’s as if the mice had been vaccinated,” Fischbach said. Their antibody response was as strong and specific as if it had been reacting to a pathogen.
“The same thing appears to be occurring naturally in humans,” Fischbach said. “We got blood from human donors and found that their circulating levels of antibodies directed at S. epidermidis were as high as anything we get routinely vaccinated against.”
That’s puzzling, he said: “Our ferocious immune response to these commensal bacteria loitering on the far side of that all-important anti-microbial barrier we call our skin seems to have no purpose.”
“We think this will work for viruses, bacteria, fungi, and one-celled parasites,” he said. If things go well, he expects this vaccination approach to enter clinical trials within two or three years.
The completed, unedited Stanford news article written by Bruce Goldman is posted at this link.