While numerous countries are reporting dengue outbreaks in 2023, one U.S. state continues to confirm new cases.
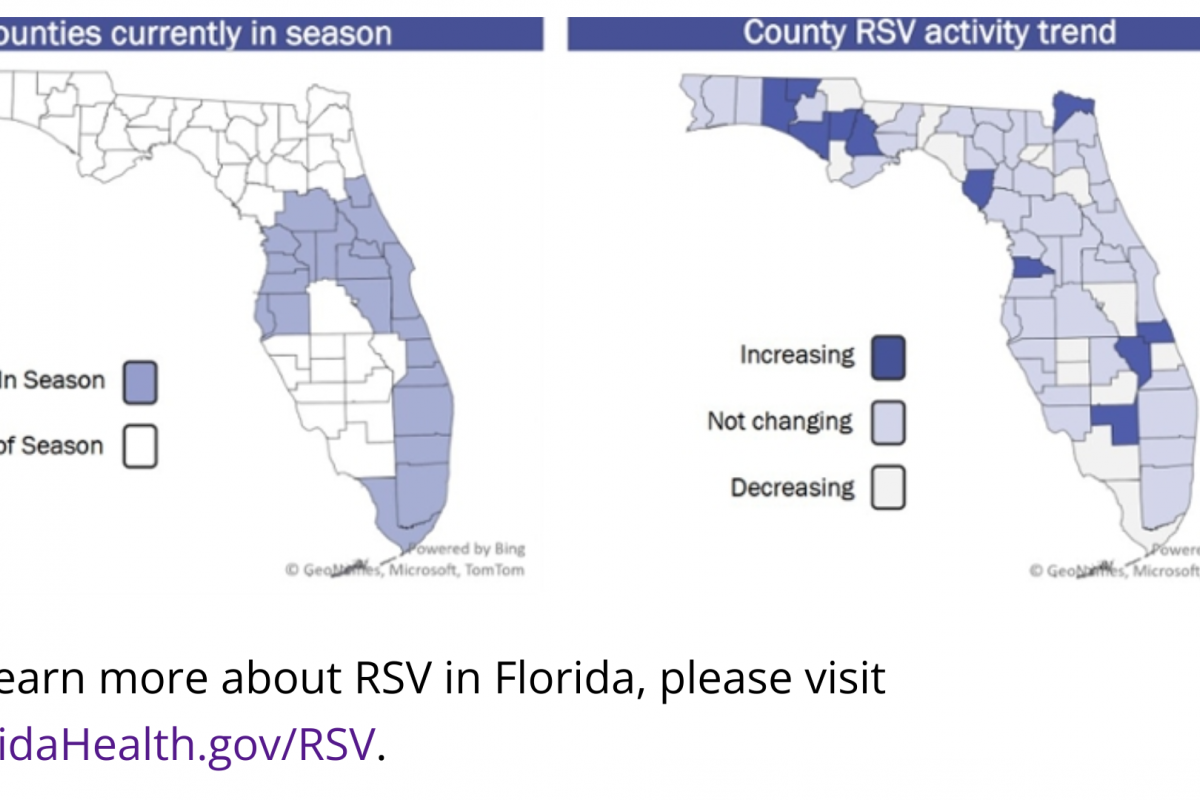
The Florida Department of Health recently reported additional dengue infections in people through the bite of Aedes species mosquitoes.
As of August 12, 2023, one case of locally acquired dengue was reported this week in Miami-Dade County. In 2023, 11 cases of locally acquired dengue have been reported.
And 14 new dengue cases were reported this week in persons with international travel.
In 2023, 204 travel-associated dengue cases have been reported in Florida. Miami-Dade Country has reported 120 of these dengue cases this year. These travelers primarily came from Cuba and Brazil.
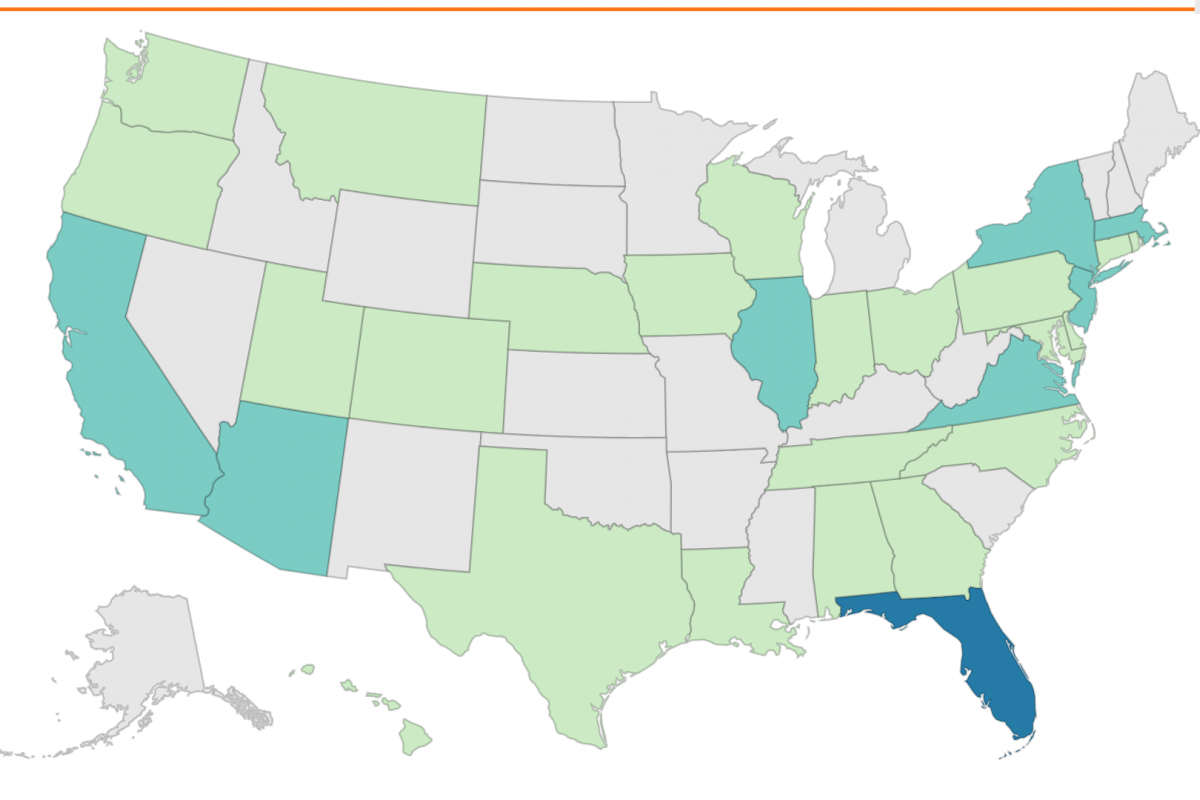
According to the U.S. CDC, 32 jurisdictions have reported a total of 513 dengue cases this year.
Dengue is a vaccine-preventable disease. The U.S. FDA, U.K., and Europe-approved Dengvaxia® (CYD-TDV) is a live attenuated tetravalent chimeric vaccine that requires pre-admission testing.