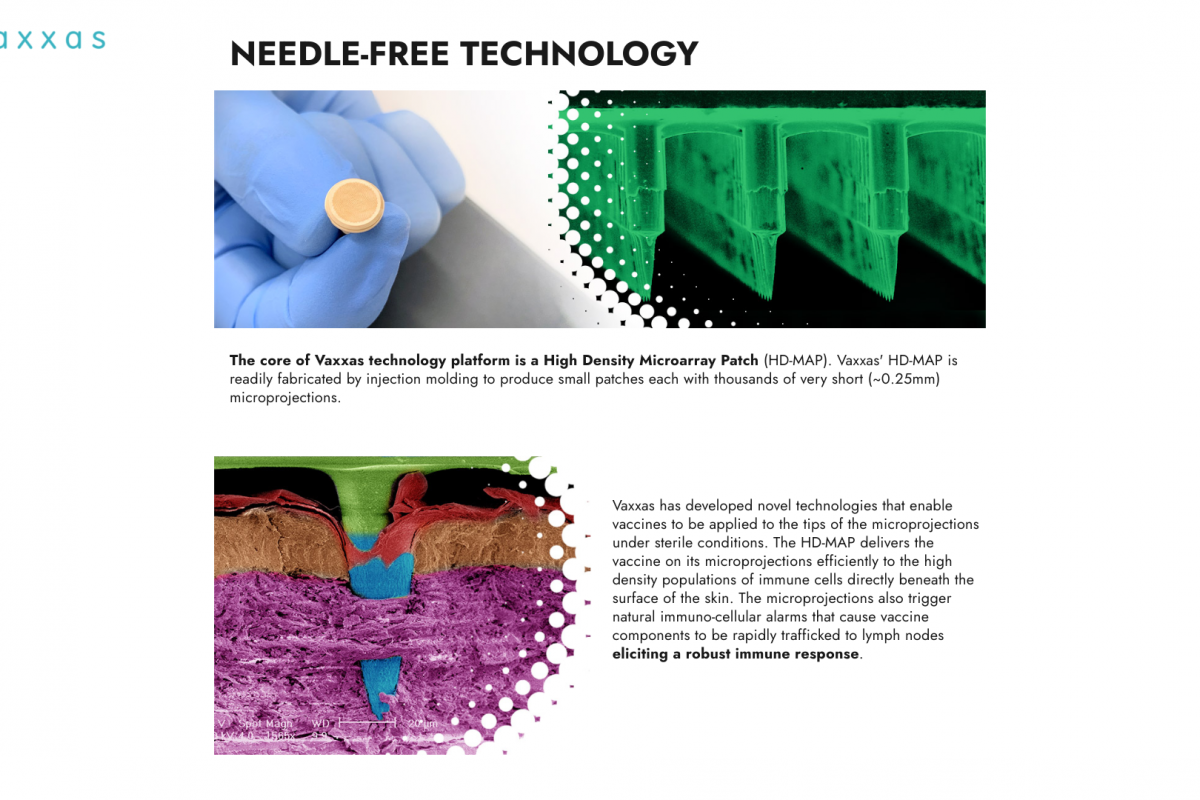
The U.S. CDC today published a Morbidity and Mortality Weekly Report (MMWR) that indicates, for the first time in ten years, human papillomavirus (HPV) vaccination initiations did not increase among adolescents in the United States.
Furthermore, coverage with one or more HPV vaccine dose among Medicaid beneficiaries declined by 3.3% in 2022 compared with coverage in 2021.
The cross-sectional analysis published on August 25, 2023, showed that HPV vaccination remained lowest among the uninsured (two of the four groups that constitute the Vaccines for Children (VFC) eligible population.
And the CDC disclosed that VFC vaccine ordering data provide additional evidence that HPV vaccination coverage might continue to decline in VFC-eligible populations.
VFC provider orders for HPV vaccines decreased 24% in 2020, 9% in 2021, and 12% during 2022 compared with 2019.
However, orders for non-HPV vaccines have rebounded to prepandemic levels (Whitlatch F, CDC unpublished data, 2023).
The HPV vaccine is the most expensive of all routinely recommended adolescent vaccines. And reimbursement levels for costs by private payers are adequate, but return margins are small for nonpediatric specialties, according to a study published in Annals of Family Medicine in July 2023.
In the U.S., the CDC's Advisory Committee on Immunization Practices recommends that children aged 11–12 years receive HPV vaccines, and some children can be started at age 9.
The CDC says HPV vaccination is the most effective way of preventing cervical cancer in women and other sexually transmitted HPV cancers.
Various HPV vaccines are available at most health clinics and pharmacies in the U.S.
This CDC report used two analyses of 2022 NIS-Teen data to examine vaccination coverage among U.S. adolescents 13 to 17 years of age.