Minimal Mpox Risk for Women and Children

According to data published by the U.S. Centers for Disease Control and Prevention (CDC), a UCLA-led multi-site study that included 45% women and 20% children, no mpox cases were reported,
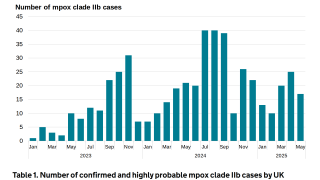
During June–December 2023, among 196 patients in the study, only three mpox cases were identified (1.5%). All cases were among men who reported having sex with multiple men in the month prior and not being vaccinated against mpox.
The CDC's MMWR revealed on June 6, 2024, that clinicians should remain vigilant for mpox virus infections and educate patients about the importance of risk reduction and JYNNEOS® vaccination.
Bavarian Nordic produces the third generation JYNNEOS® (MVA-BN®, IMVAMUNE®), a two-dose vaccine based on a live, attenuated vaccinia virus, Modified Vaccinia Ankara.
A meta-analysis of 16 studies published on April 26, 2024, revealed that the JYNNEOS vaccine effectiveness (VE) for one pre-exposure prophylactic vaccination ranged from 35% to 86%, and VE ranged from 66% to 90% for two doses.
In 2024, JYNNEOS became commercially available in the U.S. Currently, the CDC does not recommend routine immunization against mpox for the general public and has not endorsed JYNNEOS booster doses (3rd).
Our Trust Standards: Medical Advisory Committee