Immunotherapy Now Available for Treating Moderate-to-Severe Plaque Psoriasis
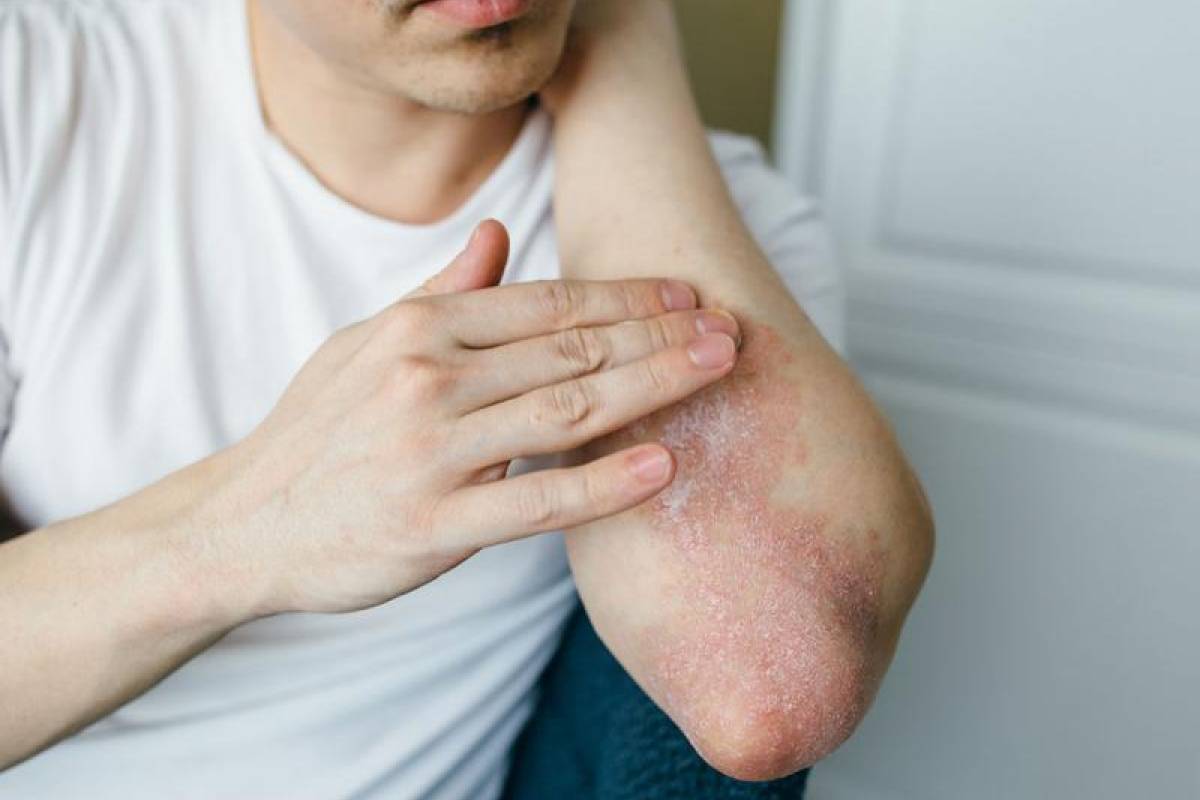
UCB announced today that BIMZELX® is commercially available for treating moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
BIMZELX is the first and only approved psoriasis treatment (humanized IgG1 monoclonal antibody) designed to selectively inhibit two key cytokines driving inflammatory processes – interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
BIMZELX (bimekizumab-bkzx) is available as an autoinjector and a pre-filled syringe.1 BIMZELX may be administered subcutaneously by a healthcare professional, or a patient may self-inject after proper training.
The U.S. Food and Drug Administration approved BIMZELX on October 17, 2023.
People living with moderate-to-severe plaque psoriasis should talk to their healthcare provider to see if BIMZELX may be right for them. However, avoid the use of live vaccines in patients treated with BIMZELX.
"While the treatment landscape for psoriasis has evolved in recent years, many unmet needs remain for the more than 7.5 million adults in the U.S. living with the disease," said Dr. Jeffrey Stark, Head of Medical, U.S. Immunology, UCB, in a press release on November 14, 2023.
"With BIMZELX now available, patients and their healthcare providers have access to a treatment that has proven to deliver rapid, complete consistently, and maintained skin clearance from the first dose."
The approval of BIMZELX is supported by data from three Phase 3, multicenter, randomized, placebo, and/or active comparator-controlled trials, which evaluated the efficacy and safety of BIMZELX in 1,480 adults.
Patients treated with BIMZELX achieved superior levels of skin clearance at week 16 compared to those who received ustekinumab (ranked secondary endpoint, BE VIVID; p<0.0001), placebo (co-primary endpoint, BE READY and BE VIVID; p<0.0001) and adalimumab (co-primary endpoint, BE SURE; p<0.001), as measured by at least a 90 percent improvement in the Psoriasis Area & Severity Index (PASI 90) and an Investigator's Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). Ranked secondary endpoints included PASI 75 at week four and PASI 100 (complete skin clearance) at week 16.
Through BIMZELX Navigate, UCB offers tailored patient support to all those with moderate-to-severe plaque psoriasis upon receiving their BIMZELX prescription. Eligible patients living in the U.S. may enroll in BIMZELX Navigate at https://www.bimzelx.com/patient-support.
The AMA's What Doctors Wish Patients Knew™ series provides physicians a platform to share what they want patients to understand about today's healthcare headlines.
The AMA wrote living with psoriasis can be incredibly frustrating and challenging. Itchy, scaly skin can hinder daily activities, affect emotional well-being, and diminish the overall quality of life.
The encouraging news is that there are strategies to ease the challenges of living with psoriasis.
Our Trust Standards: Medical Advisory Committee
