Serious Concerns Raised Regarding Monkeypox Vaccine Reduced Dosage Delivery
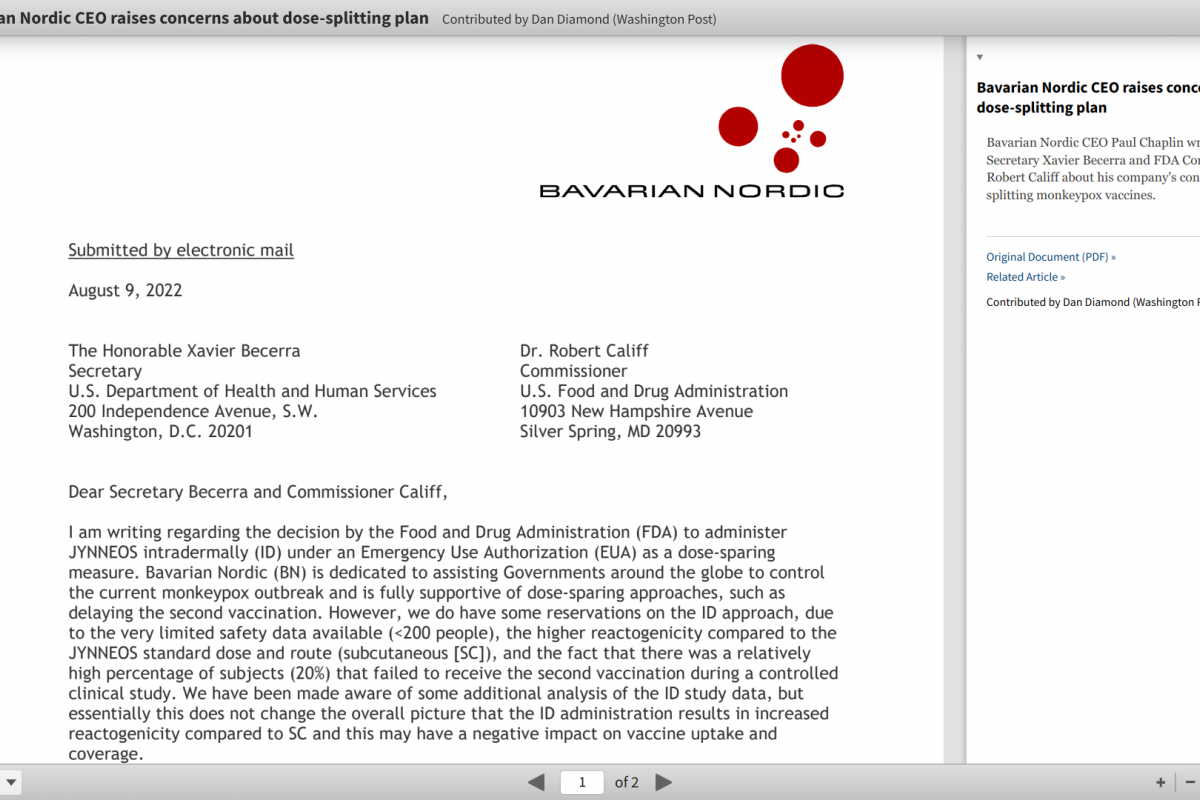
Denmark-based Bavarian Nordic CEO Paul Chaplin sent a letter to U.S. Department of Health and Human Services (HHS) Secretary Xavier Becerra and FDA Commissioner Robert Califf expressing safety concerns on August 9, 2022, about the alternative monkeypox vaccine dosage strategy the USA has launched.
Bavarian Nordic is the manufacturer of the Jynneos monkeypox vaccine currently approved by the U.S. FDA and offered to people throughout the USA.
In the letter obtained by Dan Diamond with the Washington Post, Chaplin stated, "Bavarian Nordic is dedicated to assisting Governments around the globe to control the current monkeypox outbreak and is fully supportive of dose-sparing approaches, such as delaying the second vaccination."
"However, we do have some reservations on the ID [intradermal] approach due to the very limited safety data available (<200 people)."
"We have been made aware of some additional analysis of the ID study data."
"But essentially, this does not change the overall picture that the ID administration results in increased reactogenicity compared to subcutaneous, and this may have a negative impact on vaccine uptake and coverage."
"Since last Thursday, we have been inundated with calls from U.S. government officials with questions and concerns regarding the ID administration."
"For these reasons, we believed it would have been prudent that the rollout of the FDA's EUA be supported by an implementation protocol."
"And the fact that there was a relatively high percentage of subjects (20%) that failed to receive the second vaccination during a controlled clinical study."
"We will, of course, align our responses with our colleagues at the U.S. CDC, but we believe this alignment would have been better served before any announcement to ensure the best rollout of the EUA."
To clarify the clinical aspects of these changes, the CDC published updated 'interim guidance' on August 10, 2022.
Additionally, Chaplin indicated in his letter the company might have access to more Jynneos vaccines than initially calculated.
As of August 8, 2022, the U.S. HHS Office of the Assistant Secretary for Preparedness and Response had delivered about 617,693 Jynneos vaccine doses from the Strategic National Stockpile to U.S. jurisdictions.
And on July 21, 2022, HHS confirmed the federal government would have access to more than 6.9 million Jynneos doses by mid-2023.
Other monkeypox vaccine news, including the ACAM2000 vaccine and TPOXX treatment, are posted at PrecisionVaccinations.com/Monkeypox.
Note: This letter was manually translated and curated for mobile readership.
Our Trust Standards: Medical Advisory Committee