Chronic Hepatitis B Virus Vaccine Launches 2nd Phase 2 Study

Massachusetts-based VBI Vaccines Inc. (VBI) today announced that the first patient had been dosed in a second Phase 2a/2b clinical study evaluating VBI-2601 (BRII-179), an immunotherapeutic candidate targeting chronic hepatitis B virus (HBV).
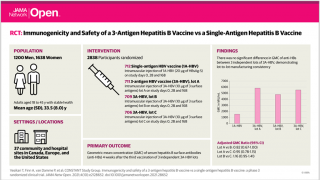
This newly announced Phase 2 study will assess VBI-2601 as an add-on therapy to the standard-of-care, nucleos(t)ide reverse transcriptase inhibitor (Nrtl) and pegylated interferon (PEG-IFN-α) therapy, which currently has a functional cure rate of approximately 9%.
A functional cure for chronic HBV infection is defined as achieving undetectable HBV surface antigen (HBsAg) levels and sustained suppression of HBV DNA.
As previously announced in April 2021, VBI-2601 is also being assessed in an additional Phase 2 study as part of a combination regimen with BRII-835 (VIR-2218), an investigational small interfering ribonucleic acid (siRNA) targeting HBV.
"Our commitment to the fight against hepatitis B includes a focus on both prevention and treatment of the disease," stated Jeff Baxter, VBI's President and CEO, in a press release issued on January 5, 2022.
"More than 290 million people are chronically infected with HBV worldwide, and, together with our partners, we are working hard to provide more patients with improved therapeutic options."
"This effort includes the assessment of new investigational treatment regimens, as in the ongoing combination study of VBI-2601 and a siRNA candidate, as well as ways to potentially improve upon functional cure rates achieved with the current standard of care, as with this newly announced Phase 2 study."
"We believe a multi-pronged approach is critical for driving innovation in the treatment of chronic HBV, and we look forward to sharing data from these two Phase 2 studies in the second half of 2022 and the first half of 2023, respectively."
Brii Biosciences is the sponsor of this newly announced Phase 2a/2b study and, with the support of VBI, has led the design and implementation of this study and the ongoing Phase 2 combination study.
Our Trust Standards: Medical Advisory Committee