Search API
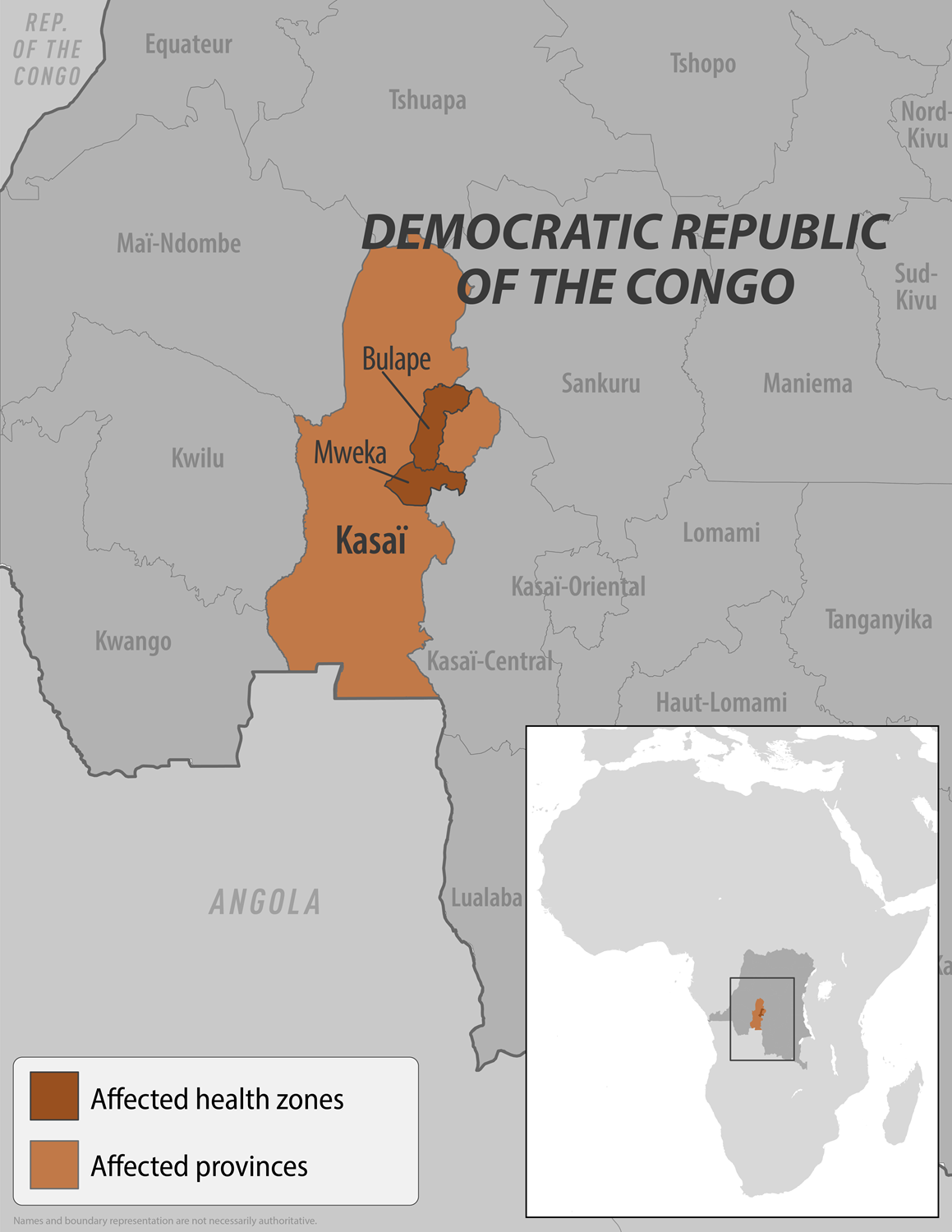
According to Reuters, the Democratic Republic of Congo (DRC) health ministry recently announced that Kasai's Bulape and Mweka zones, where the first Zaire Ebola case was reported, were placed under confinement.
In response to the DRC's 16th Ebola outbreak, and applying lessons learn, multiple travel checkpoints were put in place to prevent residents from moving in and out of the area.
On September 10, 2025, the U.S. CDC stated that there are 58 people with suspected or confirmed Ebola and 20 deaths, including four health workers.
The CDC stated it expects frequent changes to these case counts.
As of September 11, 2025, there have been no reported cases of Ebola in the United States related to this outbreak. During previous outbreaks, travel-related cases were confirmed.
The CDC has posted a Level 1 Travel Health Notice for the DRC, recommending people practice usual precautions if traveling to the DRC.
While not commercially available in the U.S., an FDA-approved vaccine and therapeutics are available for the prevention of Ebola virus infection (Orthoebolavirus zairense only). During previous outbreaks, Zaire Ebola has been shown to be a vaccine-preventable disease.
The other Ebolavirus type, Sudan, caused an outbreak in Uganda in early 2025.
Since the first outbreak of the Zaire Ebola virus was identified in 1976 in Africa, numerous outbreaks have been declared by the World Health Organization (WHO).
The WHO published a Disease Outbreak News on September 5, 2025, regarding the ongoing Ebola virus disease (EVD) outbreak in the Democratic Republic of the Congo (DRC).
As of September 4, 2025, 28 suspected cases, including 15 deaths (case fatality ratio: 54%), have been reported from three areas of the Bulape health zone (Bulape, Bulape Com, and Dikolo) and the Mweka health zone.
Although the affected district is a hard-to-reach rural area relatively far from the two main urban centres of Mbuji Mayi and Kananga, population movements between different parts of the province are frequent, especially between Bulape and Tshikapa.
Among the deaths, four are unvaccinated health-care workers.
This is the 16th Ebola outbreak in the DRC. The last EVD outbreak in the country was declared on August 15, 2022, in Beni city, North Kivu province.
In accordance with the recommendations of the Strategic Advisory Group of Experts on immunization, the Ervebo vaccine is recommended during a Zaire EVD outbreak for contacts and potential contacts of confirmed/suspected cases, as well as for frontline workers.
The DRC's Ministry of Health, with support from WHO and partners, is implementing public health response measures to contain the outbreak.
Ebola is a serious, often fatal illness transmitted to humans through close contact with the blood or secretions of infected wildlife and then spreads through human-to-human transmission.
The WHO assesses the overall public health risk posed by the current EVD outbreak as high at the national level, moderate at the regional level, and low at the global level. No travel restrictions have been issued.
As of September 8, 2025, Ebola vaccines and therapies are unavailable in the USA.

When Ebola and Marburg outbreaks have occurred over the decades, diagnosing cases has been a significant challenge for healthcare workers.
To address this essential need, Aptitude Medical Systems announced its second major partnership with the Biomedical Advanced Research and Development Authority (BARDA), with $9 million in funding to develop the Metrix Filovirus Panel.
This collaboration leverages Aptitude's next-generation molecular diagnostics platform, Metrix®, which has been advanced through a prior BARDA partnership valued at up to $61.9 million.
This rapid next-generation molecular diagnostic device aims to detect and differentiate Ebolavirus and Marburgvirus species.
"Point-of-care diagnostics are essential for effectively addressing outbreaks of high-consequence pathogens like Ebolavirus and Marburgvirus species," added JP Wang, PhD, CTO, President, and Executive Chairman of Aptitude, in a press release on July 28, 2025.
It is a small, portable platform, making it appropriate for use in remote and more traditional point-of-care settings, generating results in 30 minutes or less from venous or fingerstick blood samples.
As of July 29, 2025, there are no active Ebola and Marburg outbreaks in Africa, and Ebola vaccines and antibody therapies have been approved for use by various countries.

Through the initial five months of 2025, Zika virus (ZIKV) outbreaks continue as a significant, measurable public health concern worldwide.
In the Region of the Americas, over 12,600 Zika patients have been identified as of June 1, 2025.
Last year, 42,127 ZIka cases and two related fatalities were reported in the Americas in 2024, led by Argentina, Brazil, Bolivia, Colombia.
Foremost among public health leaders' focus is when a pregnant woman becomes infected with this mosquito-transmitted virus. While pregnant, ZIKV can induce severe defects of the fetal brain and, eventually, microcephaly in the infant.
To better understand this health risk, an Ohio State University (OSU) study published in the Proceedings of the National Academy of Sciences on May 23, 2025, reveals the biological secret to the Zika virus's infectious success.
These researchers found that Zika utilizes the host cells' own "self-care" system to clear away useless molecules, thereby suppressing the host proteins that the virus has employed to enter those cells in the first place.
They wrote in a press release on May 27, 2025, 'While these cell surface proteins are valuable for viral entry, they also have roles in producing an antiviral response. Before that can happen, the virus manipulates a process cells use to keep themselves healthy to lower the proteins' activity, clearing the way for unfettered viral infection.'
'Though other viruses, such as HIV, are known to silence host receptors that let them into cells, Zika is unusual for having at least three of its proteins that can get the job done,' said Shan-Lu Liu, senior author the study and a virology professor in the Department of Veterinary Biosciences at OSU.
"That's the most interesting part: It's amazing that not only one, but several Zika proteins can do this."
"We looked at two Zika virus strains and examined three physiologically relevant cell types. With both strains, we observed downregulation in all three cell types. It looks like this is an important mechanism," added Liu.
Although further research is needed to confirm this, there is a possibility that this mechanism is relevant to the Ebola virus, which utilizes the TIM-1 protein to access host cells, or to other pathogens in the same flavivirus family, including Zika, West Nile, yellow fever, and dengue viruses.
"The bottom line is this speaks to the co-evolution of viral-host interactions. The more important a host factor is to a virus, the more a virus is going to do to take control of it," Liu said. "Understanding these mechanisms is an important part of being prepared for emerging or reemerging viruses that cause infectious diseases."
As of June 2025, there are no Zika preventive vaccines available, and the U.S. CDC recommends pregnant women avoid visiting areas reporting Zika outbreaks.
Over the last few years, Zika cases have been reported in Puerto Rico, Costa Rica, and other tourist favorite destinations.

Few disease outbreaks have been as deeply traumatic as the 2014 Ebola outbreak in West Africa. By the time the outbreak stopped in 2016, nearly 30,000 had been infected, and 11,000 people had died.
With no vaccine available at the outbreak's start, the affected countries, Guinea, Sierra Leone, and Liberia, were unprepared to respond.
This unfortunate situation has changed with the approval of Ebola vaccines and therapeutics.
Launched in January 2021, the International Coordinating Group on Vaccine Provision, which includes the World Health Organization, UNICEF, and others, now coordinates a stockpile.
It is a Gavi-funded, globally managed reserve of Merck's ERVEBO® (rVSV-ZEBOV) vaccine that ensures rapid, equitable access to life-saving immunisation during outbreaks.
The vaccine stockpile in Switzerland is maintained at a target level of 500,000 ERVEBO doses, as the WHO’s Strategic Advisory Group of Experts on Immunization recommends.
A challenge in maintaining a stockpile is ensuring that doses are always available and do not expire.
“All Ebola outbreaks that have occurred since we had a stockpile were quickly stopped – thanks to the vaccines and rapid other response measures,” said Allyson Russell, an epidemiologist and senior programme manager in Gavi’s High Impact Outbreaks team, in a April 30, 2025 news release.
In the United States, ERVEBO® is approved by the Food and Drug Administration for preventing disease caused by the Zaire Ebola virus in individuals 12 months of age and older as a single-dose administration.
Zaire Ebola vaccines aren't effective against the other three orthoebolaviruses that cause severe disease, including the Sudan virus.
Since there are no well-controlled studies of ERVEBO in pregnancy, the U.S. CDC says, 'The risk of exposure to Ebola should be weighed against potential vaccine-related risk during pregnancy based on individual informed decisions.'
As of May 12, 2025, access to Ebola vaccines in the U.S. is restricted.

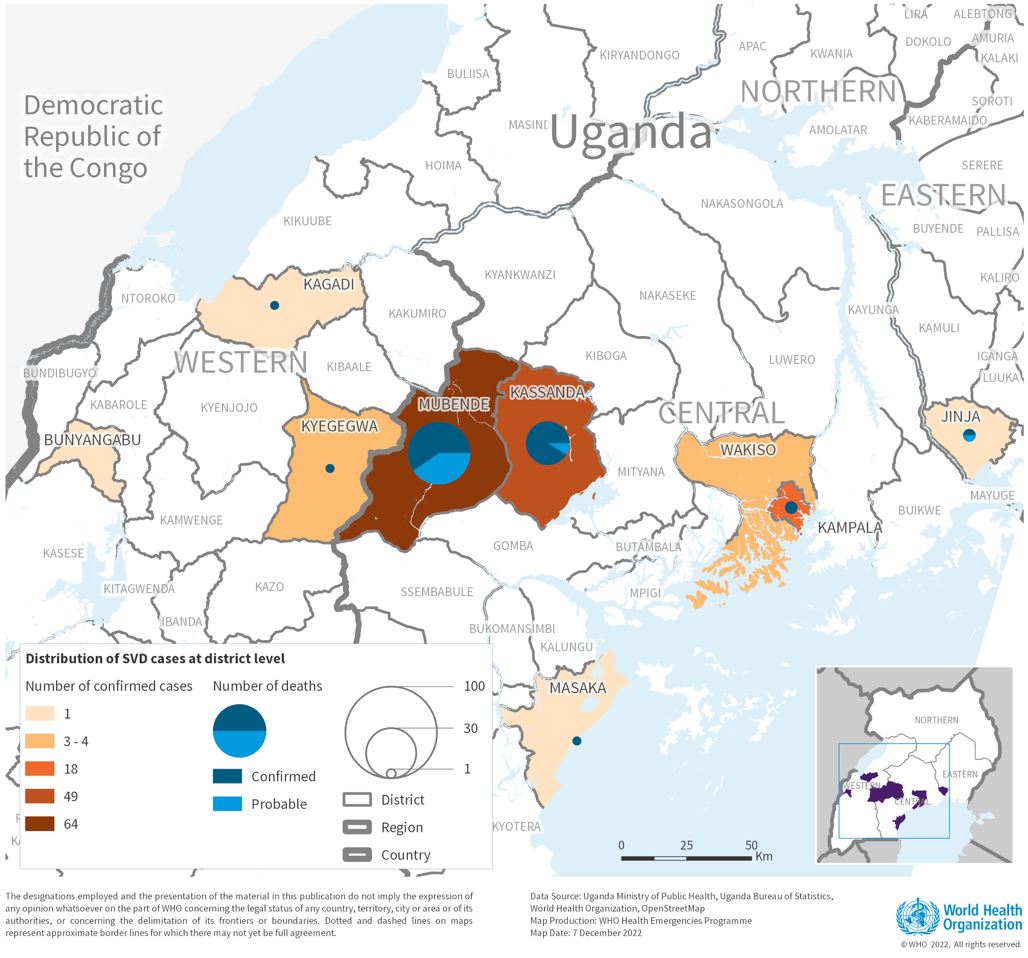
The Republic of Uganda today announced the end of the current Sudan Ebolavirus disease (SVD) outbreak, less than three months after the virus was confirmed in the capital Kampala.
This strain of Ebolaviris often causes a severe, fatal illness in infected people.
As of April 26, 2025, during this outbreak, 14 SVD cases, including 12 confirmed and two probable cases, had been reported.
And four deaths, two confirmed and two probable, occurred.
"This outbreak challenged us in new ways. It touched both urban and rural communities across the country and unfolded against the backdrop of significant global funding constraints," said Dr Chikwe Ihekweazu, Acting WHO Regional Director for Africa, in a press release on April 26, 2025.
"The response demonstrated Uganda's long-standing leadership in tackling public health emergencies. As WHO, we are extremely proud to have supported these efforts every step of the way."
Despite the absence of licensed countermeasures against this species of Ebolavirus, Sudan candidate vaccines are in various phases of clinical trials. Within four days of the government's declaration of the outbreak, a randomized clinical trial for vaccine safety and efficacy using the ring vaccination approach was launched.
In addition, the administration of Remdesivir treatment under the Monitored Emergency Use of Unregistered and Experimental Interventions protocol was initiated.
As of today, various Sudan vaccine candidates are being tested in clinical research,
Previously, Zaire Ebolavirus vaccines and therapeutics had been approved for use in Africa,
In addition to Eboa, Uganda is experiencing polio and mpox outbreaks in 2026. Vaccines for these diseases are commercially available in the U.S.
According to recent travel advisories issued by the United States government, visiting the Republic of Uganda is not encouraged in 2025.
On April 23, 2025, the U.S. Department of State reissued a Level 3: Reconsider Travel advisory for Uganda, a country home to approximately 32 million people, located in East Africa.
The State Department says visitors should exercise increased caution in Uganda due to security risks and ongoing civil unrest.
If you decide to travel to Uganda, enroll in the Smart Traveler Enrollment Program to receive alerts from the U.S. Embassy in Kampala. This free digital offering makes it easier to locate people in an emergency.
From a health perspective, Uganda is confronting multiple disease outbreaks in 2025.
Building on sustained U.S. technical support to Uganda since the first day of the 2025 Ebola outbreak, the United States government has now donated 100 vials of monoclonal antibodies (mAb) to the Uganda Ministry of Health. As a breakthrough in medical science, mAb has the potential to significantly improve survival rates in patients with Ebola.
U.S. Ambassador Popp stated in a press release on April 14, 2025, “Providing these groundbreaking treatments demonstrates the United States’ strong commitment to innovation, scientific excellence, shared prosperity, and global health security. As we respond to outbreaks like Ebola, we build stronger global health security partnerships that benefit us all.”
And will enhance the ongoing efforts to end the current Sudan Ebola Virus outbreak in Uganda, as no vaccines or therapeutics have been approved for the prevention or treatment of SVD in 2025.
Additionally, the CDC has included Uganda in its polio and mpox travel advisories and recommends pre-arrival vaccinations for several routine and travel-related diseases, such as yellow fever, mpox, and cholera.
Travel vaccines for these diseases are commercially available at clinics and pharmacies in the United States.