Search API
In response to the United States' recent naval operation off the coast of the Bolivarian Republic of Venezuela, vacationers to the southern Caribbean Islands may make alternative plans this fall season.
These vacation destinations include Trinidad and Tobago, Grenada, Barbados, and the islands of Aruba, Curaçao, and Bonaire.
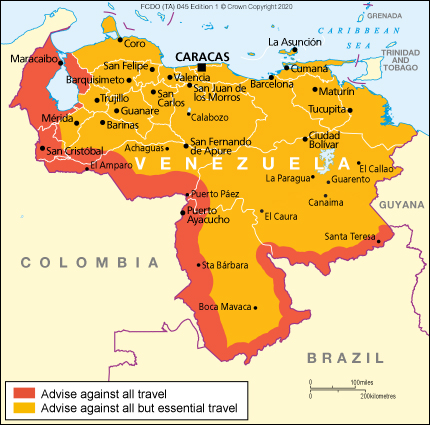
As of September 7, 2025, both the US Department of State and the UK government have updated their high-level travel advisories for Venezuela.
The US State Department writes, Do not travel to or remain in Venezuela due to the high risk of wrongful detention and civil unrest. All US citizens and Lawful Permanent Residents in Venezuela are strongly advised to depart immediately.
Additionally, no US embassy or consulate is operating in Venezuela, and the US government cannot provide routine or emergency consular services to US citizens in Venezuela.
Previously, the UK Foreign, Commonwealth & Development Office advised against travel within 80km of the Venezuela-Colombia border.
From a health perspective, if you plan to visit Venezuela in 2025, the US Centers for Disease Control and Prevention (CDC) recommends various routine and travel vaccinations before traveling to this South American country.
And since March 2025, the CDC has reported cases of Oropouche virus disease in Venezuela.
When Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) was founded in 2016, the early-stage antibiotic pipeline was stalled.
Since its inception, CARB-X has supported 115 R&D projects in 14 countries, and CARB-X product developers have made significant progress.
Recently, CARB-X awarded Baxiva AG $3 million to develop its multivalent glycoconjugate vaccine to prevent extraintestinal pathogenic Escherichia coli (ExPEC) infections.
Baxiva's proprietary conjugation platform streamlines the development of multivalent vaccines targeting the serotype-specific polysaccharides of Gram-negative bacteria, including capsule and O antigens.
The multivalent vaccine targets the most common serotypes associated with invasive ExPEC infections.
Multivalent vaccines are designed to prevent infections caused by multiple strains or types of a single pathogen. The glycoconjugate formulation combines polysaccharide (sugar) from a pathogen's surface with a carrier protein to enhance immune response and therefore the effectiveness of the vaccine.
Escherichia coli is the leading cause of urinary tract infections, a frequent cause of neonatal sepsis, and is among the leading causes of antimicrobial resistance-associated deaths globally.
"Vaccines are a powerful tool in the global effort to prevent infections and curb the spread of antimicrobial resistance," said Erin Duffy, PhD, R&D Chief of CARB-X, in a press release on August 21, 2025.
"Baxiva's multivalent glycoconjugate vaccine project explores a range of novel polysaccharide antigens in vaccine candidate solutions, addressing a critical unmet need in infection prevention."
"We are excited to welcome Baxiva into the CARB-X portfolio and support the advancement of their platform."
The E. coli bacteria cause most urinary tract infections (UTIs).
As of August 2025, UTI vaccines are unavailable in the United States.

The U.S. National Institute of Allergy and Infectious Diseases has initiated a Phase 1 clinical trial for a new ferritin-based nanoparticle vaccine aimed at preventing infection by Epstein-Barr virus (EBV).
Since May 2025, this study has been conducted at the National Institutes of Health Clinical Center in Maryland, and began to evaluate the safety of a 3-dose vaccination regimen of an adjuvanted EBV gH/gL/gp42-ferritin nanoparticle vaccine with or without gp350-ferritin, with 750 participants.
This represents a significant milestone in the development of an EBV vaccine, as there is currently no FDA-approved vaccine available for this virus.
The U.S. CDC states most people will get infected with EBV in their lifetime, especially in childhood, and will not have symptoms. EBV is known to cause infectious mononucleosis and has been associated with several autoimmune diseases and cancers.
According to a NIH media release in 2022, "A vaccine that could prevent or reduce the severity of infection with the Epstein-Barr virus could reduce the incidence of infectious mononucleosis and might also reduce the incidence of EBV-associated malignancies and autoimmune diseases," said NIAID Director Anthony S. Fauci, M.D.
According to ClinicalTrials.gov, this study's expected completion date is October 2027.