Search API
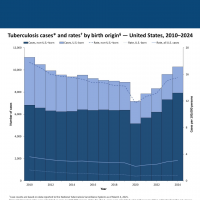
The French Republic's overseas department and region of Réunion today reported a serious spike in Chikungunya cases. Over the last week, 4,156 new cases were reported.
This data indicates a 16% increase in Chikungunya cases compared to the previous week.
Furthermore, emergency department activity increased from 78 admissions the previous week to 128 during March 10-16, 2025.
During early March, Réunion health authorities announced two virus-related deaths.
Since this Chikungunya outbreak began in August 2024, 13,594 cases, 15 serious cases (eight adults and seven newborns) have been reported, setting an unfortunate record for a French department or region.
Réunion's southern and western municipalities are the most affected. Le Tampon remains the most affected municipality.
However, this mosquito-transmitted virus has increased sharply in Possession, Saint Andrew, Saint Paul, and Saint Suzanne.
As part of the Mascarene Islands, Réunion is a vacation destination for many travelers. It is located east of Africa, Madagascar, and southwest of Mauritius.
France has issued a Level 4 emergency for Réunion to alert visitors of this health emergency, and the U.S. CDC issued a Level 2 Advisory regarding La Réunion's Chikungunya outbreak.
To reduce the impact of this outbreak, Valneva SE and the Agence Régionale de Santé La Réunion recently announced an agreement to deliver at least 40,000 IXCHIQ® vaccine doses starting in early April 2025. The French government funded this purchase.
In the U.S. and various countries, Chikungunya vaccines are approved and available at travel clinics and pharmacies. They are recommended for international travelers visiting endemic countries such as La Réunion.
As sexually transmitted diseases continue to spread in 2025, a new vaccine candidate may reduce the number of chlamydia cases in the United States. Current programs to prevent infection rates from rising have proven insufficient, highlighting the urgent public health need for a vaccine.
Sanofi announced today that the U.S. Food and Drug Administration has granted a fast-track designation to Sanofi's mRNA vaccine candidate for preventing chlamydia infection.
The chlamydia vaccine candidate has been designed to protect against primary genital tract infection and reinfection by the bacterium Chlamydia trachomatis.
In 2020, there were 129 million worldwide cases of chlamydia among adults and adolescents, with the highest rates of infection among younger people.
Jean-François Toussaint, Global Head of Vaccines R&D, stated in a press release on March 26, 2025, "Millions of people currently live with undiagnosed chlamydia, including asymptomatic infection that can also cause severe long-term health effects if left untreated."
"Antibiotics to treat chlamydia have not been successful in controlling rising infection rates. With our program, we aim to make chlamydia a preventable disease through vaccination."
Following a promising preclinical program, Sanofi is planning a phase 1/2 randomized clinical study to evaluate the immunogenicity and safety of the chlamydia vaccine candidate in adults aged 18 to 29.
This clinical study is due to start in the coming days.
Chlamydia, caused by the bacterium Chlamydia trachomatis, is a common bacterial infection of the reproductive tract with consequences for developing infertility and pregnancy complications.
Although chlamydia can be treated with antibiotics when diagnosed, over 80% of chlamydia cases are asymptomatic, meaning there is a significant risk that infections go unrecognized, leading to untreated cases and unintentional transmission.
A study published by the Annals of Family Medicine on March 24, 2025, indicates that many women are not receiving guideline-adherent treatment in primary care settings. For example, the time to treat chlamydia was longer for patients aged 50-59 years (time ratio relative to those aged 20-29 years = 1.61; 95% CI, 1.12-2.30).
The development of this vaccine candidate is part of the Translational Science Hub, a partnership with the Queensland Government, Griffith University, and the University of Queensland that connects world-class researchers in Queensland, Australia, with Sanofi scientists in France and the U.S.
As of late March 2025, the FDA, the United Kingdom, and the European Medicines Agency have not approved a vaccine to prevent chlamydia infections.
With about 100 different types of pneumococcal bacteria infecting people, innovative vaccines are needed to reduce various illnesses, including pneumonia, meningitis, and bloodstream infections.
For example, in Europe, 17,700 confirmed invasive pneumococcal disease (IPD) cases were reported in the European Union/European Economic Area in 2022.
To address this disease, Merck announced today that the European Commission (EC) has approved CAPVAXIVE® (V116), designed to help protect adults against IPD and pneumococcal pneumonia.
CAPVAXIVE targets the serotypes causing most IPD cases in adults and includes eight unique serotypes not covered by other approved vaccines.
This EC decision authorizes the approval of CAPVAXIVE (Pneumococcal 21-valent Conjugate Vaccine) in all 27 European Union (EU) member states, including Iceland, Liechtenstein, and Norway.
“By focusing on the serotypes that have been responsible for an increasing proportion of adult invasive pneumococcal disease cases, CAPVAXIVE allows us to offer protection specifically designed for adults,” said Dr. Paula Annunziato, senior vice president, infectious diseases and vaccines, Global Clinical Development, Merck Research Laboratories, in a press release on March 26, 2025.
“We are proud to bring CAPVAXIVE to adults in Europe who may benefit from its broad protection and are eager to continue working with regulatory authorities to expand CAPVAXIVE availability worldwide.”
The EC approval is based on safety and immunogenicity data from the Phase 3 STRIDE clinical program.
As of late March 2025, various pneumococcal vaccines are offered in the United States at clinics and pharmacies.
Note: This VBT news article was updated for trademark on March 27, 2025.

Without a preventive vaccine available in the United States, an innovative treatment for uncomplicated urinary tract infections (uUTIs) has been approved.
New treatments are needed as the number of uUTIs caused by drug-resistant bacteria has increased, which can result in higher treatment failure rates.
GSK plc today announced that the U.S. Food and Drug Administration (FDA) has approved Blujepa, a first-in-class oral antibiotic with a novel mechanism of action that is GSK's infectious diseases portfolio.
Blujepa is approved for the treatment of female adults (≥40 kg) and paediatric patients (≥12 years, ≥40 kilograms) with uUTIs caused by Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii complex, Staphylococcus saprophyticus, and Enterococcus faecalis.
GSK's Chief Scientific Officer, Tony Wood, commented in a press release on March 25, 2025, "The approval of Blujepa is a crucial milestone with uUTIs among the most common infections in women.
"We are proud to have developed Blujepa, the first in a new class of oral antibiotics for uUTIs in nearly three decades."
uUTIs are the most common infection in women, impacting up to 16 million women in the U.S. annually.
Over half of all women are affected by uUTI in their lifetime, with approximately 30% suffering from at least one recurrent episode, which can cause significant patient burden, including discomfort and restriction of daily activities.
Two other products have been approved for use.
Pivya™ (Pivmecillinam) is an extended-spectrum penicillin antibiotic. Pivya targets penicillin-binding protein-2 in the cell wall of gram-negative bacteria, and has been available in Europe.
ORLYNVAH™ is a U.S. FDA-approved novel oral penem antibiotic for treating uUTIs caused by the designated microorganisms Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis in adult women.
While an oral UTI vaccine is available in several countries in 2025, Uromune™ (MV140) is unavailable in the U.S.
However, international travelers can request a vaccine appointment using this Vax-Before-Travel link.

When visiting countries in the Americas in 2025, travelers to areas where the Zika virus is known to be present are at risk of infection.
However, it can be challenging to determine the exact level of risk in each Zika outbreak.
According to the U.K. Travel Health Pro, those who stay for extended periods in regions where the Zika virus is common face a higher risk. Nevertheless, even short-term visitors may be exposed to the virus, such as in the Federative Republic of Brazil.
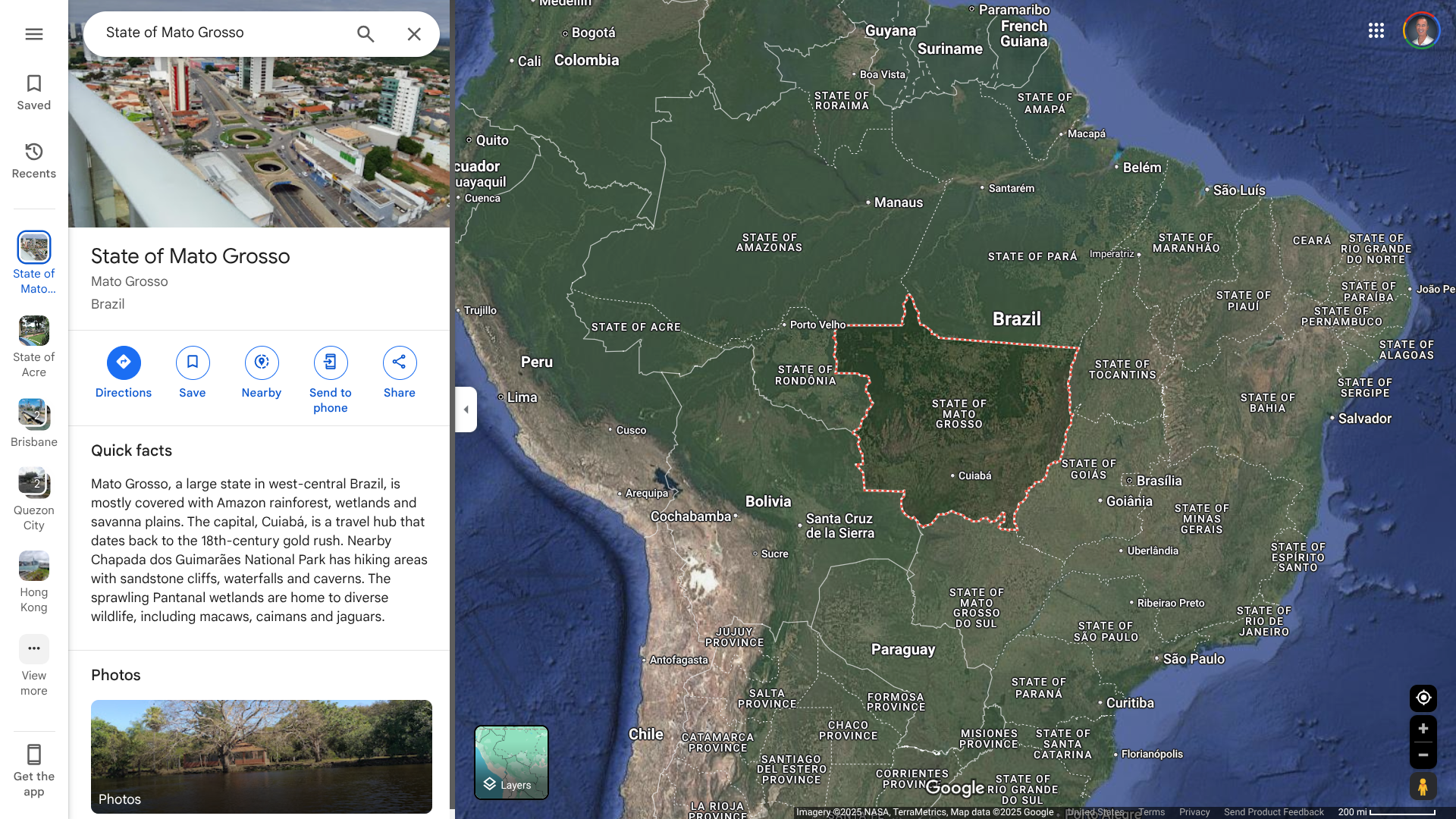
As of March 25, 2025, about 2,556 Zika cases have been reported in Brazil since the beginning of 2025.
Most of these cases have been reported in Mato Grosso, São Paulo, Acre, and Rio Grande do Norte. Mato Grosso is a mountainous state in west-central Brazil, mainly covered by the Amazon rainforest.
Just to the south, the Argentine Republic has reported 433 Zika cases this year.
Previously, the U.S. CDC removed a Level 2 Travel Advisory in 2021 regarding Argentina's Zika outbreak.
To the north, in the United States, no Zika cases have been confirmed in 2025.
Last year, the U.S. CDC reported 19 non-congenital Zika cases among U.S. residents, primarily in Puerto Rico.
With millions of international travelers expected to visit these areas in 2025, Zika vaccines will not be available. However, several Zika vaccine candidates are conducting clinical research and aspiring for future U.S. FDA approval.
While this respiratory disease is generally related to camel interactions in the Kingdom of Saudi Arabia, clinical efforts to produce a Middle East Respiratory Syndrome (MERS) vaccine have been elusive.
Since April 2012 and as of mid-March 2025, six World Health Organization regions have reported 2,618 cases of MERS, including 945 deaths, a significant case-fatality rate.
To address this need, CEPI announced, on March 25, 2025, a $2.6 million investment in moving a promising vaccine candidate into preclinical trials.
This new investment, developed by Newark, DE-based Uvax Bio, an early-stage vaccine technology company spun out of The Scripps Research Institute, is based on proprietary protein nanoparticle technology, 1c-SApNP®, licensed from Scripps Research.
The technology is already being tested against other infectious diseases, including HIV, where an in-human trial is ongoing.
Dr. Kent Kester, Executive Director of Vaccine R&D, CEPI, commented in a press release, “Uvax Bio’s unique vaccine could help strengthen our response to future MERS outbreaks while informing us of the vaccines being developed against other coronaviruses.”
Uvax’s novel vaccine design uses tiny protein “nanoparticles” to closely resemble or mimic the size and shape of the MERS coronavirus.
Uvax Bio has analyzed viral structures and designed the technology to present enhanced antigens —parts of the virus that trigger an immune response—in a multilayered scaffold layout. This design offers stability and allows for as many as twenty antigens to be presented at once, which could help provide strong protection by generating both antibody and T-cell immunity.
The 1c-SApNP® technology is also unique as it has been combined with a process called ‘glycan trimming.’
Here, sugar molecules—called glycans—that would generally cover the MERS virus are shortened in the nanoparticle virus-mimicking vaccine design. This could expose additional sites on the antigen surface, enhancing the immune response.
In addition to the vaccine candidate, several MERS vaccines are in clinical development addressing this zoonotic disease with an unknown source in 2025.

A substantial Chikungunya outbreak is occurring throughout France's overseas department of La Réunion Island, which has spiked since early 2025, with 8,600 cases recorded.
To protect the residents of La Réunion, Valneva SE today announced an agreement with support from the local public health agency, the Agence Régionale de Santé La Réunion, to deliver 40,000 IXCHIQ® vaccine doses starting in early April, with an option to provide more.
IXCHIQ® is the world’s first licensed chikungunya vaccine to address this significant unmet medical need
This supply of doses, paid for by the French authorities, is in line with the recommendation of France’s national public health agency, the Haute Autorité de Santé, to prioritize vaccination for adults aged 65 and over with co-morbidities.
Furthermore, IXCHIQ® remains available for purchase in France (mainland and overseas), as well as throughout Europe, the United States, and the U.K.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release on March 24, 2025, “Chikungunya outbreaks spread rapidly, so it is crucial to vaccinate as many people as possible to help contain the virus."
"We have the capacity to supply more doses and will continue working closely with Agence Régionale de Santé La Réunion to manage this outbreak locally and prevent its spread to other regions through international travel.”
La Réunion is located east of Africa and Madagascar and is a favorite vaccination destination for international travelers.
Furthermore, Valneva recently announced an agreement with the Serum Institute of India, the world’s largest manufacturer of vaccines by number of doses, enabling the vaccine supply throughout Asia.
In the U.S., Chikungunya vaccines are commercially offered at travel clinics and pharmacies in 2025.

According to the World Health Organization, Dengue fever continues to be endemic in Southeast Asia and the Western Pacific in 2025.
This includes popular tourist destinations for Hong Kong people, such as the Philippines, Thailand, Indonesia, Malaysia, Singapore, and in mainland China, such as Guangdong.
As of March 20, 2025, the Centre for Health Protection (CHP) in Hong Kong confirmed international visitors are arriving infected with Dengue. Seven imported Dengue cases have been reported this year, primarily from Indonesia (3).
In 2024, 161 were imported (75 from Mainland China, 19 from Indonesia) and five locally transmitted cases.
In 2023, there were 62 imported cases of Dengue were recorded in Hong Kong.
The CHP writes, 'Members of the public returning from areas affected by DF should apply insect repellent for 14 days upon arrival in Hong Kong. If feeling unwell, seek medical advice promptly and provide travel details to a doctor.'
Hong Kong is a special administrative region of China, with over 7 million residents, and welcomes millions of international visitors yearly.
As of March 24, 2025, Hong Kong and China are not listed in the U.S. CDC's Level 1 - Practice Usual Precautions, Travel Health Advisory regarding the global Dengue outbreak.
Outside of the United States, a second-generation Dengue vaccine is offered in about 40 countries.