Search API
With the continued confirmations of mpox infections in various countries, the U.S. FDA-approved vaccine is now being evaluated for at-risk, vulnerable populations.
Bavarian Nordic A/S announced that on June 26, 2025, the initiation of the first of two clinical trials designed to support approval and use of the MVA-BN® (JYNNEOS) mpox/smallpox vaccine in infants under 2 years of age, pregnant, and breastfeeding women.
Both studies are conducted in the Democratic Republic of Congo, the epicentre of the ongoing mpox outbreak in Africa, where infants and pregnant women remain highly vulnerable to the sexually transmitted mpox virus.
Paul Chaplin, President & CEO of Bavarian Nordic, commented in a press release, “These new studies will fill the gap by providing important data about the use of MVA-BN ... which could help support a label expansion for MVA-BN to include the most vulnerable populations.”
Furthermore, these studies are part of the PregInPoxVac research project, which includes a phase 2 trial of MVA-BN in children aged 2-11 years. Topline results from this trial (NCT06549530) are anticipated in the third quarter of 2025.
Currently, JYNNEOS is commercially offered in the United States at various clinics and pharmacies.
According to the U.S. CDC, the majority of clade II mpox cases in the U.S. continue to be in people who are not vaccinated or who have received only one dose of JYNNEOS.
As of June 1, 2025, approximately 35,000 mpox infections had been reported in the United States.

The mosquito-transmitted West Nile virus (WNV) has been actively spreading throughout 14 Louisiana parishes since 2002 and continues to do so in 2025.
On June 25, 2025, the Louisiana Department of Health (LDH) confirmed Louisiana’s first human case of WNV of the 2025 mosquito season. This case was confirmed in an individual from Livingston Parish, located east of Baton Rouge and north of New Orleans, who was hospitalized due to complications from the infection.
In 2024, there were 57 confirmed human cases of WNV in Louisiana, including three deaths.
In 2024, 1,466 WNV cases were confirmed in 49 states in the USA, led by Texas with 176 cases.
The LDH says WNV can cause mild to severe illnesses. While most people infected with West Nile virus develop no symptoms, about 20% of infected individuals develop West Nile fever, a flu-like illness characterized by symptoms that can include fever, headaches, body aches, nausea, and rashes. About 1 in 150 people who are infected with WNV develop a severe illness that can affect the brain, spinal cord, and nerves, which may even cause paralysis or death.
WNV is an international concern.
For example, according to a research program by the UK Health Security Agency and the Animal and Plant Health Agency, WNV genetic material was detected in mosquitoes in Britain for the first time in 2025.
As of June 27, 2025, vaccine candidates to protect people against WNV have not been approved in the United States.
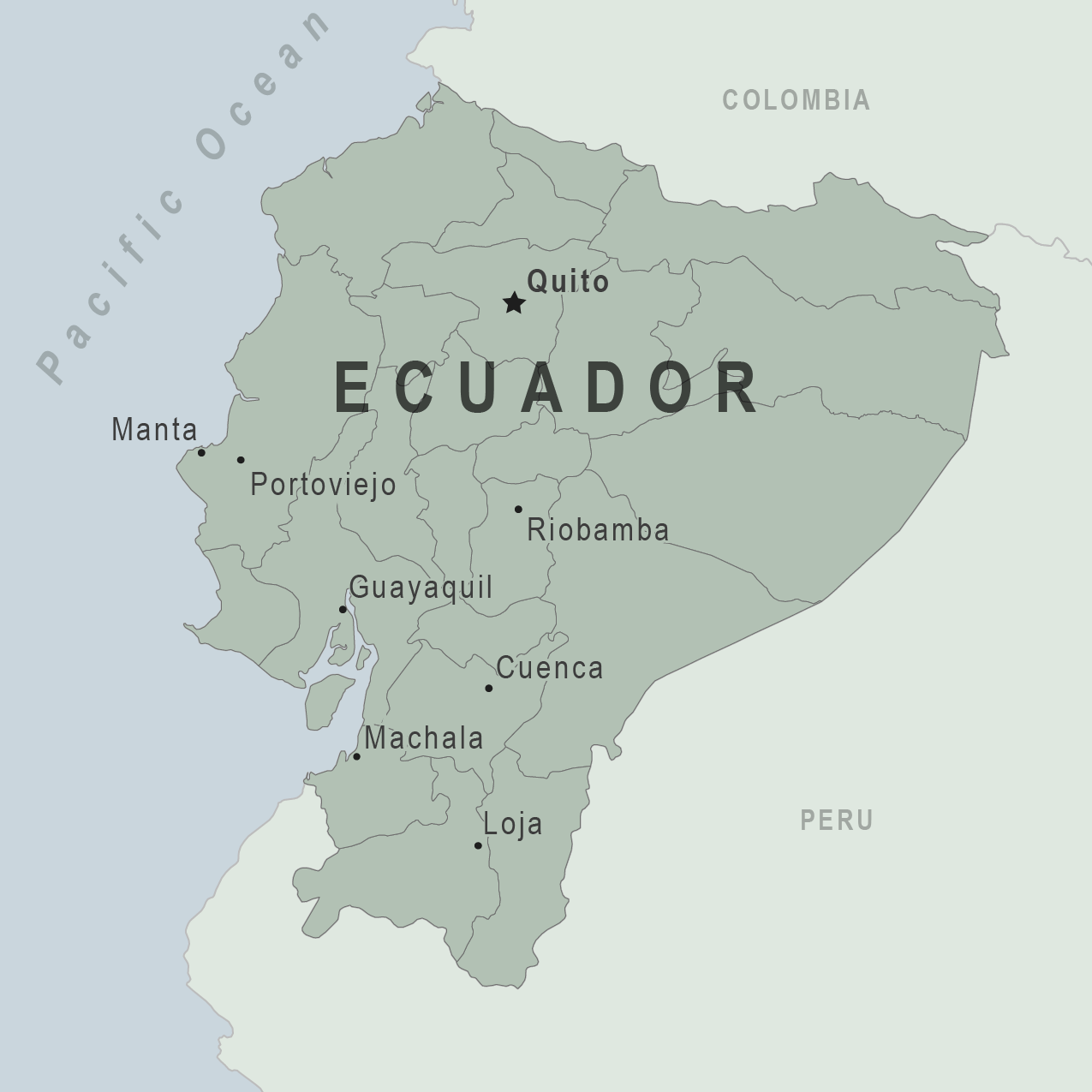
The Ecuadorian Ministry of Public Health reported 10 confirmed fatal cases of yellow fever and eight related fatalities as of June 28, 2025.
These patients were primarily from the provinces of Morona Santiago and Zamora Chinchipe.
Current U.S. CDC Travel Health Advisory recommendations include that yellow fever vaccination is recommended for travelers ≥9 months old going to areas below 7,550 ft elevation, east of the Andes Mountains, in the provinces of Morona-Santiago, Napo, Orellana, Pastaza, Sucumbíos, Tungurahua*, and Zamora-Chinchipe.
These areas are where virus-carrying mosquitoes are found.
Vaccine is generally not recommended for travel limited to areas below 7,550 ft elevation, west of the Andes Mountains, in the provinces of Esmeraldas,* Guayas, Los Ríos, Manabí, Santa Elena, Santo Domingo de los Tsáchilas, and designated areas in the provinces of Azuay, Bolívar, Cañar, Carchi, Chimborazo, Cotopaxi, El Oro, Imbabura, Loja, and Pichincha.
And for the cities of Guayaquil or Quito (the capital), or the Galápagos Islands.
The Plurinational State of Bolivia recently declared a National Health Emergency in response to a measles outbreak in ten communities.
The government reported on June 25, 2025, that Health Minister Maria Renee Castro highlighted the severity of measles, describing it as a highly contagious viral infection that can lead to severe complications, including pneumonia, encephalitis, and even death, particularly among malnourished or immunocompromised children.
"The most affected group is children under 10 years old; therefore, we declared Red Alert in Santa Cruz and enabled vaccination centers in 24-hour health facilities in all 56 municipalities until weekends,"
She stressed, "It is essential that parents get their children vaccinated. The country has 500,000 doses, specifically for girls and boys."
As of June 26, 2025, the U.S. CDC has included Bolivia in its recent measles and yellow fever Travel Health Advisories, recommending that travelers speak with a travel vaccine expert before visiting Bolivia.
These travel alerts are essential, as about 1 million people visited Bolivia last year.
The first malaria vaccine recommended by the World Health Organization (WHO) is expected to become significantly less expensive in Africa soon.
This news is essential as most of the malaria-endemic countries have the highest rates of infections and deaths, which are found in Africa.
Bharat Biotech International Limited (BBIL) and GSK plc today announced that Bharat Biotech will reduce the price of the RTS,S vaccine (Mosquirix™) by more than half, to less than $5, progressively by 2028.
This price reduction is driven by process improvements, expanded production capacity, cost-effective manufacturing, and minimal profit margins. Bharat Biotech has invested over $200 million in new, higher-output manufacturing facilities, product development, and technology transfers.
Dr. Krishna Ella, Executive Chairman of BBIL, said in a press release, "Through this historic announcement, we aim to change the course of malaria burden for millions of children and families. For us, this is more than a cooperation, it’s a promise..... At Bharat Biotech, we believe technology must deliver on three essentials: safety, affordability, and accessibility."
"With this collaboration, we are turning this belief into real impact, to ensure life-saving vaccines reach the communities that need them most.”
With Gavi’s support, 12 endemic countries in Africa will have introduced RTS,S through routine immunisation programmes by the end of 2025.
As of June 26, 2025, neither malaria vaccine is available in the United States.

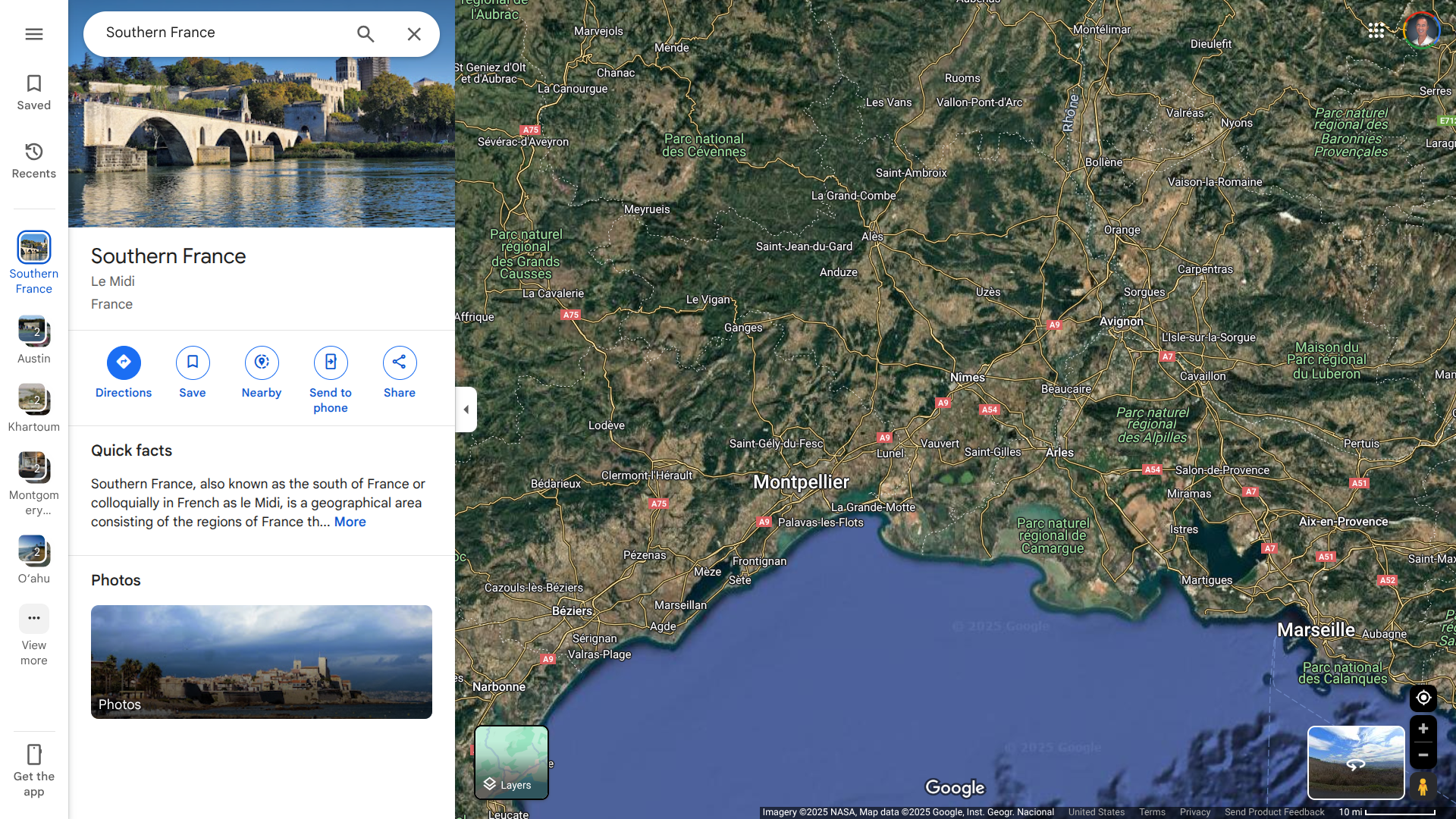
Sante Publique France reported today that a total of eight locally acquired cases of chikungunya have been confirmed in southern France since late May 2025.
Cases in France's mainland were reported in Occitane (Hérault and Gard), Provence-Alpes-Côte d'Azur (Var and Bouches-du-Rhône), Auvergne-Rhône-Alpes (Drôme), and on the island of Corse (Corse-du-Sud).
Although declining as of June 25, 2025, the number of chikungunya cases that have traveled to Réunion and the Indian Ocean remains high. It contributes to the early appearance of indigenous transmission.
Additionally, 645 imported cases of chikungunya have been reported over the past nine weeks.
The government says, 'This number of episodes has never been observed in France before.'
Public Health France reiterates the importance of protective measures against mosquito bites, the control of larval breeding sites, and the use of protective vaccination, if appropriate.
Overall, global coverage for vaccines against diphtheria, tetanus, pertussis, measles, polio, and tuberculosis nearly doubled from 1980 to 2023.
However, this long-term trend masks recent challenges.
To achieve 90% global coverage for life-course vaccines, we must accelerate progress to reach current estimates of zero-dose children.
As of June 24, 2025, most zero-dose children remain concentrated in regions with various resource constraints that limit the availability of vaccination services.
This analysis, published in The Lancet, discloses that, as of 2023, more than 50% of the 15.7 million global zero-dose children reside in eight countries: Nigeria, India, the Democratic Republic of the Congo, Ethiopia, Somalia, Sudan, Indonesia, and Brazil.
These researchers wrote, 'findings underscore the crucial need for targeted, equitable immunisation strategies, strengthening primary health-care systems, and adapting to local contexts are essential to advancing coverage.'