Search API
The World Health Organization (WHO) recently announced that the Federal Democratic Republic of Nepal, a country located high in the Himalaya Mountains in southeast Asia, has eliminated rubella as a public health problem.
Rubella, or German measles, is a vaccine-preventable disease that is a highly contagious viral infection. It is severe for pregnant women as infection can lead to a range of lifelong and debilitating congenital disabilities, says the WHO.
"Nepal's success reflects the unwavering commitment of its leadership, persistent efforts of the health care workers and volunteers, and unstinting support of engaged and informed communities, for a healthy start for babies and a future free of rubella disease," said Dr Catharina Boehme, Officer-In-Charge WHO Southeast Asia, in a press release on August 18, 2025.
Unfortunately, this disease eradication success has not translated to the Japanese Encephalitis (JE), a flavivirus transmitted by mosquitoes that is often contracted from infected pigs and sheep.
As of August 2025, JE infections are the leading cause of viral encephalitis in 24 countries in the WHO Southeast Asia and Western Pacific/Oceania Regions, exposing more than 3 billion people to infection risks.
In Nepal, Japanese Encephalitis has been a significant public health concern since 1978, especially in the Terai region, an area that borders India.
JE is also a vaccine-preventable disease. The WHO indicated in August 2025 that Nepal's national JE vaccination coverage surpasses 95%. The government integrated JY vaccinations beginning in 2015.
However, as of August 19, 2025, the Health Ministry's data show 33 JE cases and three fatalities this year.
In 2024, 23 people died, and 80 others were infected with the JE virus.
Traditionally, JE vaccination hasn't been routinely recommended for short-term travelers to Nepal.
The WHO and the U.S. CDC recommend that international travelers visiting Nepal consider vaccination. Proven JE vaccines are available in the United States, commercially offered at various travel clinics and pharmacies in 2025.
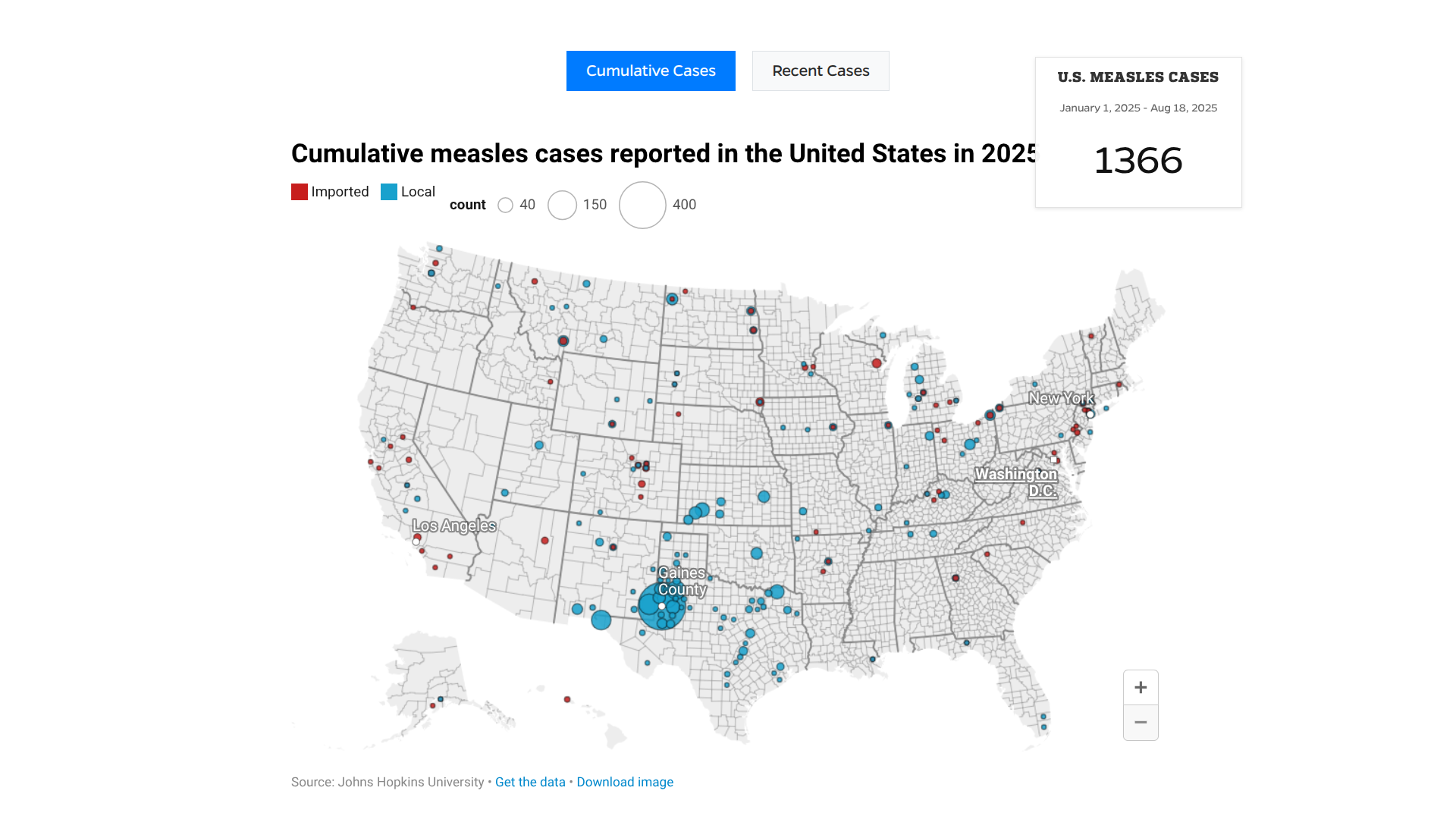
The Pan American Health Organization (PAHO) recently urged countries in the Americas to strengthen immunization activities as measles cases continue to rise in the region.
As of August 8, 2025, a total of 10,139 confirmed measles cases and 18 related deaths have been reported across ten countries, representing a 34-fold increase compared to the same period in 2024.
Countries with the highest case numbers include Canada (4,548 cases), Mexico (3,911 cases), and the United States (1,356 cases).
In Mexico, most deaths have occurred in indigenous people between 1 and 54 years of age.
However, in the U.S., the state of Texas recently declared its measles outbreak had ended.
The current outbreaks are associated with two genotypes of the measles virus and linked to low vaccination coverage, with 71% of cases occurring in unvaccinated individuals.
One genotype has been identified in outbreaks across eight countries, particularly among Mennonite communities in Canada, the United States, Mexico, Belize, Argentina, Bolivia, Brazil, and Paraguay.
In 2024, coverage with the first dose of the measles, mumps, and rubella (MMR) vaccine in the region reached 89% (two percentage points higher than in 2023), while the second dose increased from 76% to 79%.
"Measles is preventable with two doses of a vaccine, which is proven to be very safe and effective. To stop these outbreaks, countries must urgently strengthen routine immunization and conduct targeted vaccination campaigns in high-risk communities," said Dr. Daniel Salas, Executive Manager of the Special Program for Comprehensive Immunization at PAHO, in a press release on August 15, 2025.
Worldwide, measles cases continue to be reported in numerious countries in 2025. To alert international travelers, the U.S. CDC published a Level 1 Travel Health Advisory in May, recommending MMR vaccination before visiting outbreak areas.
Before 2006, chikungunya fever infections were rarely identified in the United States. However, starting in 2014, cases of chikungunya began to appear among U.S. travelers returning from affected regions in the Americas.
Local transmission of the virus has since been documented in Florida, Texas, Puerto Rico, and the U.S. Virgin Islands.
As of August 19, 2025, there have been 50 reported travel-related cases in the U.S.
Last year, 199 cases were confirmed.
The U.S. Centers for Disease Control and Prevention (CDC) has highlighted several countries where travelers are acquiring this mosquito-borne disease.
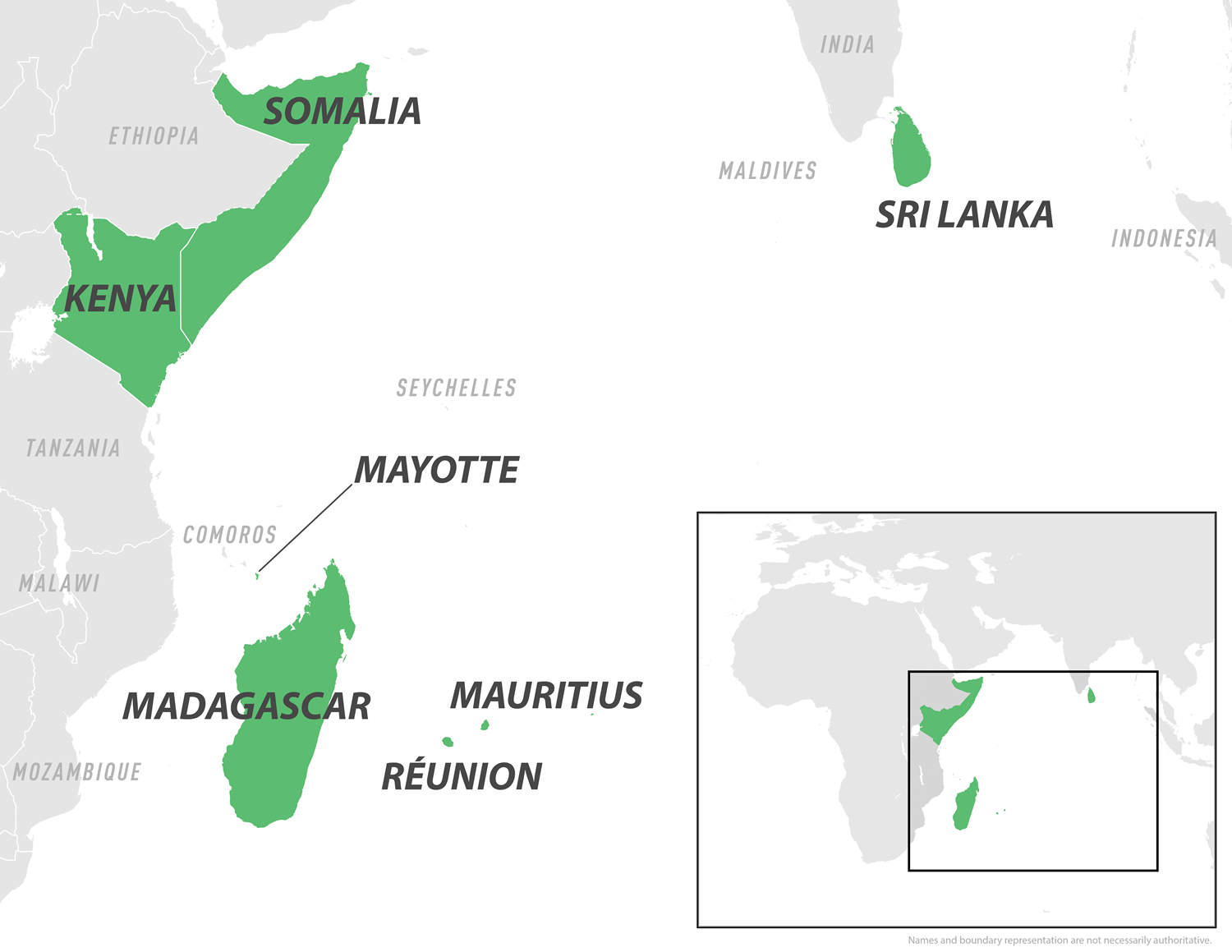
The CDC has issued a chikungunya travel health notice for outbreaks in Bolivia, China, Kenya, Madagascar, Mauritius, France, the Department of Mayotte and Réunion, Somalia, and Sri Lanka.
According to the Pan American Health Organization (PAHO), Brazil is leading all countries in the region with over 204,000 cases in 2025.
The CDC recently wrote that international travelers can protect themselves by preventing mosquito bites. And, if you are pregnant, you should reconsider travel to the affected areas, particularly if you are close to delivering your baby.
"The CDC recommends chikungunya vaccination for travelers going to areas with current outbreaks, and also for people traveling to regions or countries with elevated risk—particularly if they'll be staying for six months or longer," Jeri Beales, MSN, RN, BS, informed Vax-Before-Travel.
"This is especially true for individuals who are older and have chronic conditions like diabetes."
"The list of countries and regions with ongoing outbreaks and elevated risks changes frequently, so be sure to seek advice from a travel clinic or physician's office that specializes in travel health before your trip," added Beales, with Destination Health Travel Clinic in the greater Boston, MA area.
Currently, two chikungunya vaccines are approved for use and commercailly in the U.S. at certified travel clinics and pharmacies.
As summer draws to a close in August 2025, millions of beachgoers flock to New Jersey's extensive coastline. While many vacationers enjoy the waves and surfing at the shore, this year, mosquito bites may become a popular topic of conversation.
According to news posted today, the New Jersey Departments of Health (NJDOH) and Environmental Protection are investigating a case of malaria in a resident of Morris County with no international travel history.
NJDOH wrote on August 18, 2025, that it is possible the resident was infected by a virus-carrying mosquito with malaria in New Jersey.
If confirmed, this would be the first known locally acquired case of malaria in New Jersey since 1991.
"While risk to the general public is low, it's important to take the necessary precautions to prevent locally acquired malaria in New Jersey. The most effective ways are to prevent mosquito bites in the first place and to ensure early diagnosis and treatment of malaria in returning travelers,” said Acting Health Commissioner Jeff Brown, in a press release."
“Anyone traveling to countries with widespread malaria should take appropriate steps to prevent malaria while traveling and monitor for symptoms.”
Malaria is a mosquito-borne disease caused by a parasite transmitted by certain mosquitoes and is widespread in many tropical and subtropical countries.
For example, the state of Florida has reported numerious travel-related malaria cases involving travelers visiting Cuba in 2025.
While approved for use in Africa, malaria vaccines are currently unavailable in the United States.
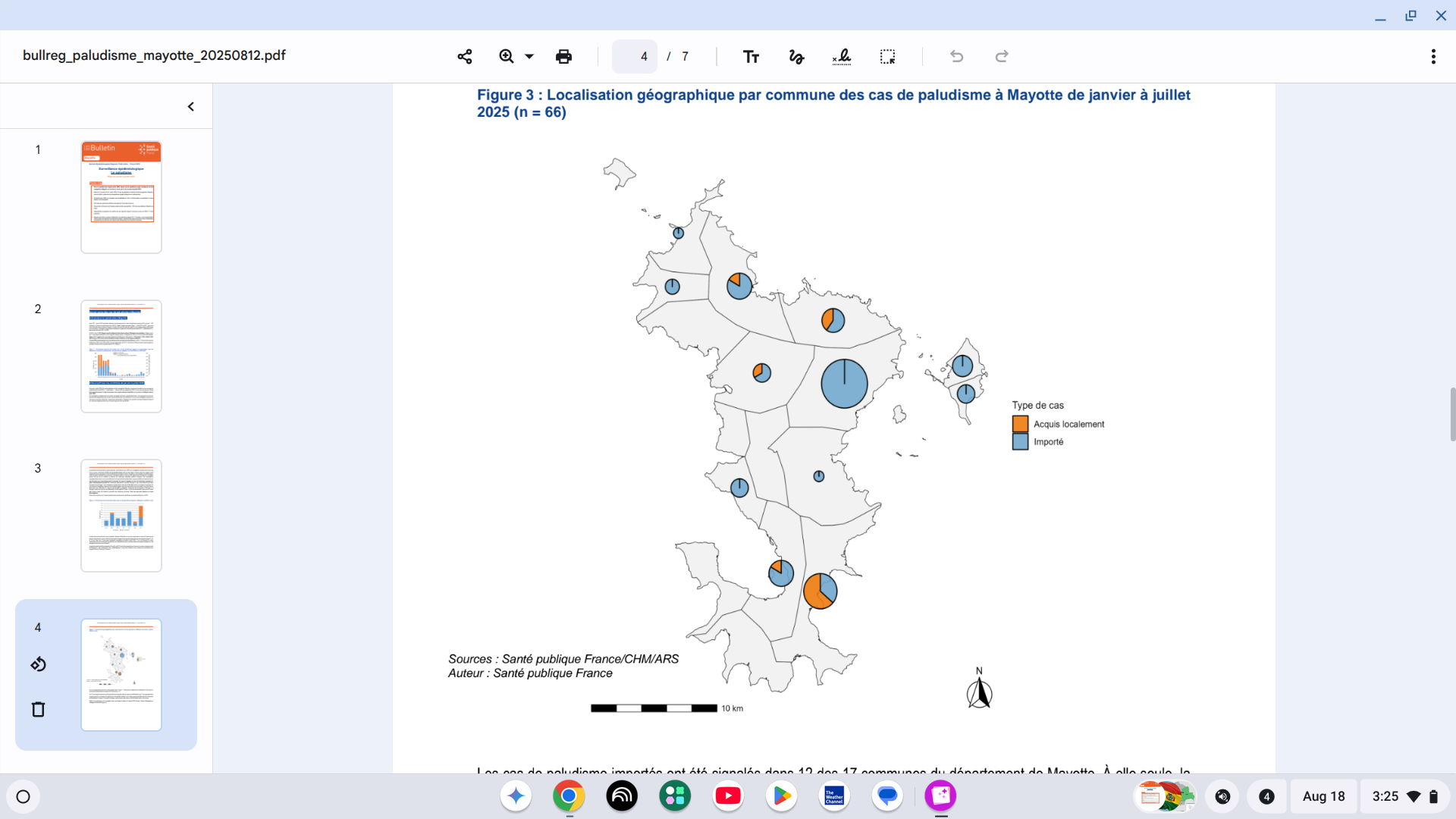
The French Health Ministry recently reported that twelve cases of locally acquired malaria were recorded throughout the Mayotte Department in 2025. These are the first local cases since July 2020.
Additionally, 54 cases of imported malaria were recorded across the main island of Mayotte.
As of August 14, 2025, there have been a total of 66 cases of malaria reported in Mayotte this year.
Several imported cases, mainly from neighbouring countries in Africa, have been reported in previous years.
While available in Africa, malaria vaccines are not offered in Mayotte.
In addition to the this mosquito-borne disease, Mayotte has been significantly impacted by the Chikungunya virus in 2025. Both travel-related and locally acquired cases have been confirmed in all areas.
Unlike malaria, Chikungunya vaccines are approved and available in 2025.
These vaccines are needed in various Indian Ocean countries, such as Mauritius, Mayotte, Réunion, and Sri Lanka.
The U.S. CDC stated recently in a Level 2 Travel Health Advisory that, if eligible, Chikungunya vaccination is recommended before visiting outbreak areas in August 2025.
Since first detected in the Plurinational State of Bolivia in 2015, the Chikungunya virus has increasingly spread throughout this South American Country.
As of August 18, 2025, the Pan American Health Organization (PAHO) reported over 4,700 CHIKV cases and one related fatality.
Last year, the PAHO reported 505 CHIKV cases.
To alert international travelers planning visits to Bolivia, specifically the department of Santa Cruz, the U.S. CDC recently issued a Level 2 Travel Health Advisory.
The CDC stated that if you are pregnant, you should reconsider travel to the affected areas, particularly if you are close to delivering your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
Newborns infected in this way or by a mosquito bite are at risk for severe illness, including poor long-term outcomes.
The CDC says vaccination is recommended for travelers who are visiting an area with a chikungunya outbreak. Two chikungunya vaccines are approved for use in the United States.
These CHIKV vaccines are commercially offered at certified retailers.
The U.S. State Department recommends enrolling in the Smart Traveler Enrollment Program when visiting Bolivia to receive digital alerts and make it easier to locate you in an emergency.

The U.S. National Institute of Allergy and Infectious Diseases has initiated a Phase 1 clinical trial for a new ferritin-based nanoparticle vaccine aimed at preventing infection by Epstein-Barr virus (EBV).
Since May 2025, this study has been conducted at the National Institutes of Health Clinical Center in Maryland, and began to evaluate the safety of a 3-dose vaccination regimen of an adjuvanted EBV gH/gL/gp42-ferritin nanoparticle vaccine with or without gp350-ferritin, with 750 participants.
This represents a significant milestone in the development of an EBV vaccine, as there is currently no FDA-approved vaccine available for this virus.
The U.S. CDC states most people will get infected with EBV in their lifetime, especially in childhood, and will not have symptoms. EBV is known to cause infectious mononucleosis and has been associated with several autoimmune diseases and cancers.
According to a NIH media release in 2022, "A vaccine that could prevent or reduce the severity of infection with the Epstein-Barr virus could reduce the incidence of infectious mononucleosis and might also reduce the incidence of EBV-associated malignancies and autoimmune diseases," said NIAID Director Anthony S. Fauci, M.D.
According to ClinicalTrials.gov, this study's expected completion date is October 2027.