Search API
Madison County Public Health (MCPH) recently announced its first recorded human case of eastern equine encephalitis (EEE) in Madison County, New York.
On September 22, 2025, MCPH stated in a press release that it had been notified of a confirmed case in a Madison County resident by the Wadsworth Center and is currently investigating. The individual is currently hospitalized for EEE infection.
This disease is often fatal (30%), and many patients who survive EEE experience neurologic impairment.
"Temperatures are getting cooler, and although we are seeing a significant decrease in mosquitoes, they remain not only a nuisance, but a potential health threat. Residents should continue to take steps to prevent mosquito bites to reduce the risk of mosquito-borne disease infection," MCPH Director Eric Faisst stated.
In July 2025, the New York State Department of Health notified MCPH of a mosquito pool that tested positive for EEE. From 1971 through 2024, 12 individuals in New York were diagnosed with EEEV; seven of them died.
About one year ago, a Ulster County resident died following an EEE infection.
This is concerning news since Madison County is located adjacent to Syracuse, New York, which has a population of over 600,000.
EEE is a rare but severe viral disease spread by infected mosquitoes that can affect people and horses. People of all ages are susceptible to infection. While most people bitten by an infected mosquito will not develop symptoms, severe cases may begin with the sudden onset of headache, high fever, chills, and vomiting.
There is no commercially available human vaccine or treatment for EEE. The best protection is to prevent mosquito bites, says MCPH.
The World Health Organization (WHO) recently published its 58th situation report on the multi-country outbreak of mpox, detailing the global epidemiological situation and providing an update on the continuing emergency in Africa, where over 90% of cases have been confirmed. Both clades of the monkeypox virus (MPXV) continue to circulate.
As of September 19, 2025, the WHO reported 59 countries across all WHO regions reported a total of 3,780 confirmed cases, including 15 deaths (case fatality ratio 0.4%) in August.
The Eastern Mediterranean and European regions reported an increase in cases in August compared to July 2025.
Nineteen countries in Africa have reported active mpox transmission in the past six weeks.
Clade IIb MPXV continues to be mostly reported in West Africa, Central African countries report both clade Ia and clade Ib MPXV, and East African countries report clade Ib MPXV.
Even though the WHO has downgraded the global Mpox situation, it continues to provide guidance and technical support to countries on targeted vaccination strategies. WHO is supporting countries on planning for the use of dose-sparing options (single dose or intradermal fractional dosing) of MVA-BN vaccine.
More than 1.1 million MVA-BN vaccine doses have been administered.
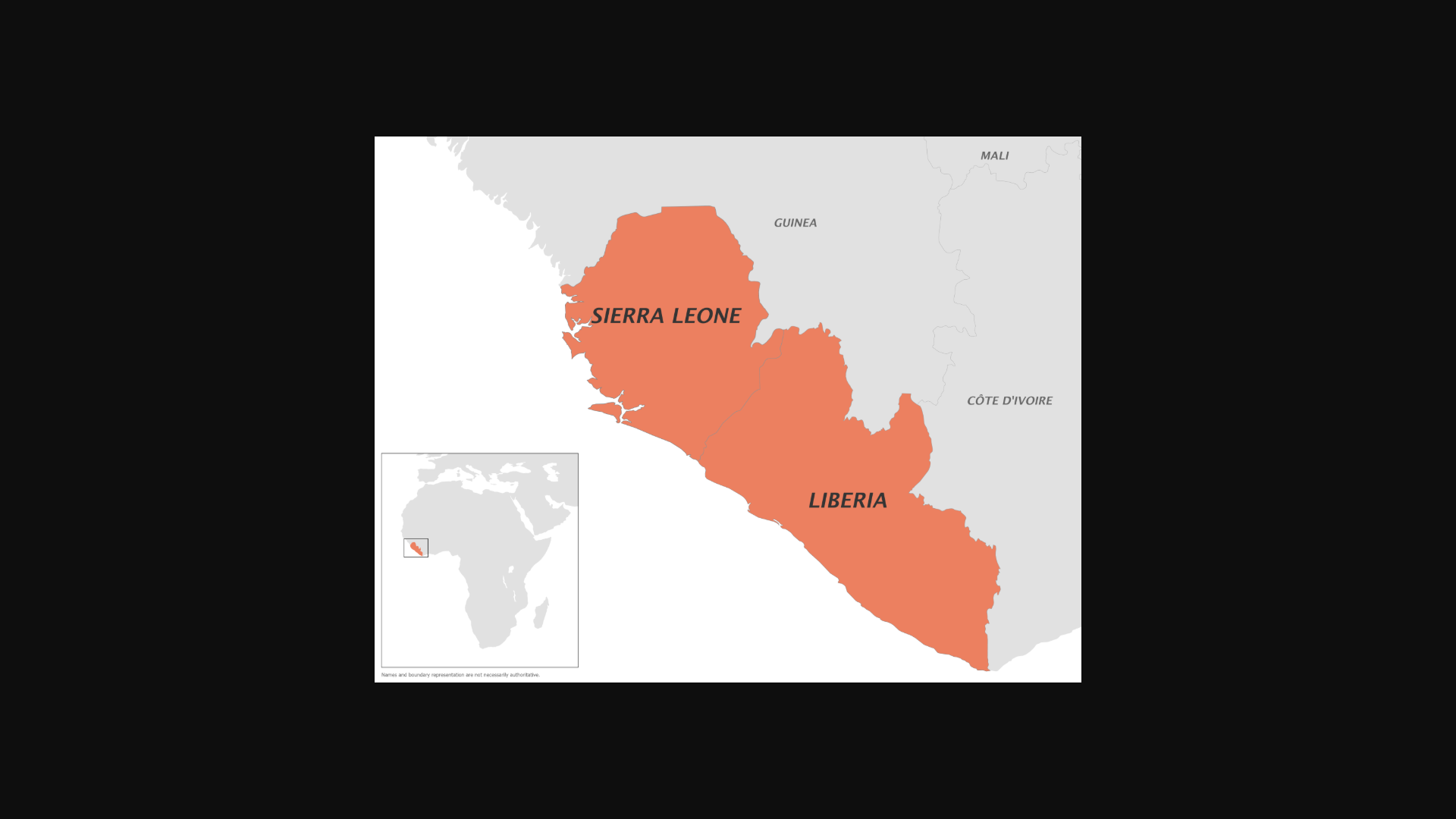
Mpox vaccination activities have started in 11 countries with the MVA-BN vaccine (Angola, Côte d'Ivoire, the Central African Republic, Democratic Republic of the Congo, Kenya, Liberia, Nigeria, Rwanda, Sierra Leone, South Africa, and Uganda). Most countries are implementing a single-dose strategy targeting population groups at high risk of exposure to the human-to-human transmitted virus.
The U.S. CDC recently stated that clade II mpox has become endemic in Liberia and Sierra Leone.
To ensure the United States has an ample supply of vaccines, Emergent BioSolutions Inc. recently announced a $56 million contract to supply ACAM2000® (Smallpox and Mpox (Vaccinia) Vaccine, Live) to the U.S. government.
Deliveries are expected to begin in September.
In the United States, the MVA-BN (JYNNEOS) vaccine remains available at health clinics as of September 22, 2025.
An unprecedented situation arose during the summer of 2025, with the first locally acquired cases of Chikungunya virus disease detected as early as June, notably affecting the Grand Est region for the very first time.
Since the beginning of 2025, France has reported 480 cases of Chikungunya virus infection.
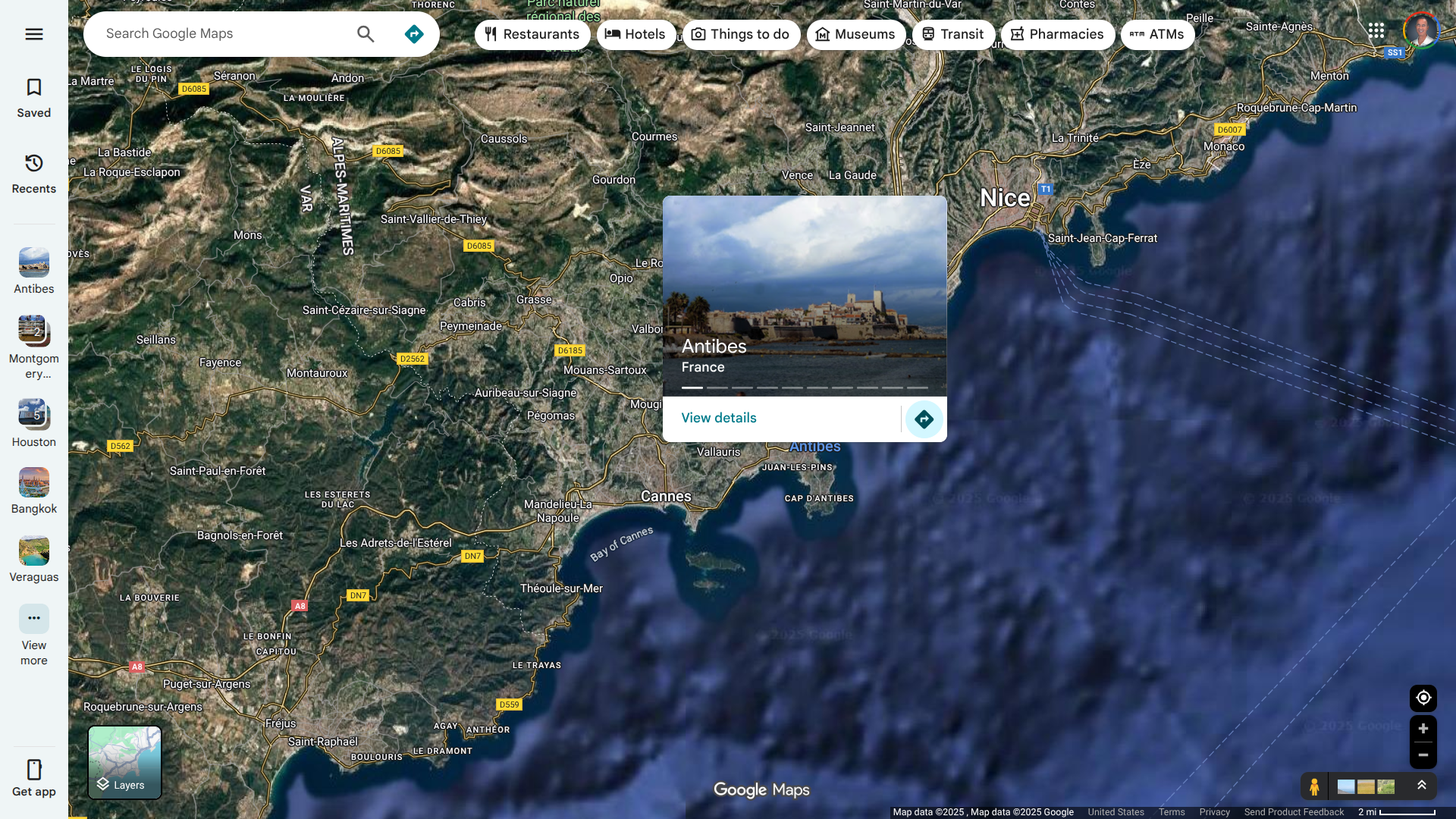
In mid-September 2025, the French Health Ministry reported 97 new locally acquired cases of Chikungunya in thirty-eight active clusters. The largest cluster is located in Antibes and consists of 87 cases.
This area of France's Mediterranean coast is a popular vacation destination, located between Cannes and Nice.
The European Centre for Disease Prevention and Control (ECDC) stated on September 12, 2025, that Chikungunya is not endemic in mainland Europe, with the majority of cases being travel-related.
However, when environmental conditions are favourable, in areas where the Aedes aegypti mosquito is established, viraemic travel-related cases may lead to local transmission of the virus, as demonstrated by the sporadic events of chikungunya virus transmission since 2007.
For more information on locally acquired Chikungunya cases in Europe, refer to the ECDC's seasonal surveillance report.
In addition to avoiding mosquito bites, the French and U.S. governments have approved vaccines for use in areas with Chikungunya outbreaks.
As of September 22, 2025, the U.S. CDC advises international travelers to speak with a travel vaccine expert regarding immunization options before traveling abroad.
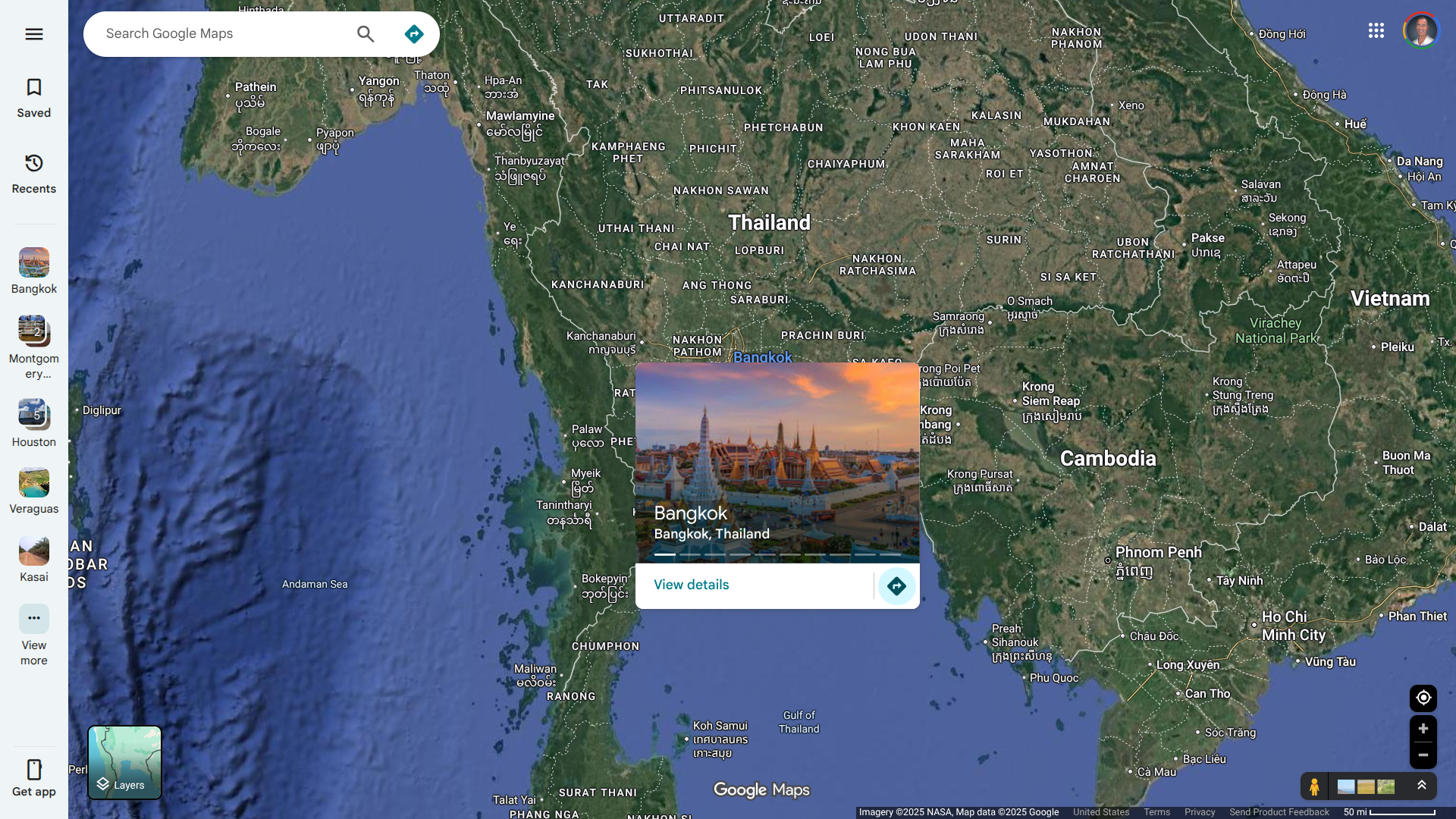
The European Centre for Disease Prevention and Control (ECDC) recently reported that health authorities in Bangkok, Thailand, issued an alert regarding human rabies cases following the detection of sick animals in some regions of the city.
From 2024 to the first quarter of 2025, a total of eight human deaths from rabies were reported in the Kingdom of Thailand.
The ECDC wrote on September 19, 2025, the alert included recommendations included if a person has been bitten or scratched by a dog, cat, bat or other mammal in an area where rabies virus is circulating, they are advised to seek medical help immediately, as the timely prophylaxis in the event of exposure to a potentially infected animal is of utmost importance and knowledge of the epidemiological situation is vital to decide on appropriate post-exposure measures.
Treatment consists of local wound care, vaccination, and passive immunisation with immunoglobulin, if indicated.
To be effective, treatment has to be administered as soon as possible after exposure.
On September 22, 2025, the Public Health Veterinary Office, in collaboration with the Prawet District Office, the Wat Pak Bo Public Health Service Center 22, and public health volunteers, conducted a rabies vaccination campaign.
The ECDC wrote that the probability of infection for travellers in Thailand's endemic areas is very low if basic preventive measures are followed, such as avoiding contact with wild and domestic animals, including pets.
In 2024, Bangkok welcomed approximately 30 million international visitors.
Those planning outdoor activities in high-risk zones or remote areas should receive an individual risk assessment and be offered pre-exposure rabies vaccination, if appropriate.
The U.S. CDC writes that rabies vaccines are typically available throughout most of Thailand.
However, please consult with a travel vaccine provider to determine whether you should receive pre-exposure vaccination before travel.
In the United States, most rabies cases are associated with bat bites, rather than those caused by dogs or cats.
The China CDC recently published its Notifiable Infectious Diseases Report, which highlights various disease cases and related fatalities.
As of September 19, 2025 (10.46234/ccdcw2025.203), several vaccine-preventable diseases that international travelers should be aware of before departing for China were disclosed.
According to the UK TravelHealthPro, when planning a trip to China in 2025, travelers should be up to date with routine vaccination courses and boosters as recommended in the UK.
These vaccinations include, for example, the measles-mumps-rubella vaccine and the diphtheria-tetanus-polio vaccine.
Those who may be at increased risk of an infectious disease due to their work, lifestyle choices, or specific underlying health problems should be up to date with additional recommended vaccines.
Seperately, the U.S. Centers for Disease Control and Prevention (CDC) recently published a Level 2 - Practice Enhanced Precautions, Travel Health Notice focused on a Chikungunya fever (CF) outbreak in southeastern China.
Chikungunya is a viral infection spread by mosquitoes. It causes a flu-like illness and can lead to severe joint and muscle pains, which may persist for months or even years. But it is rarely fatal.
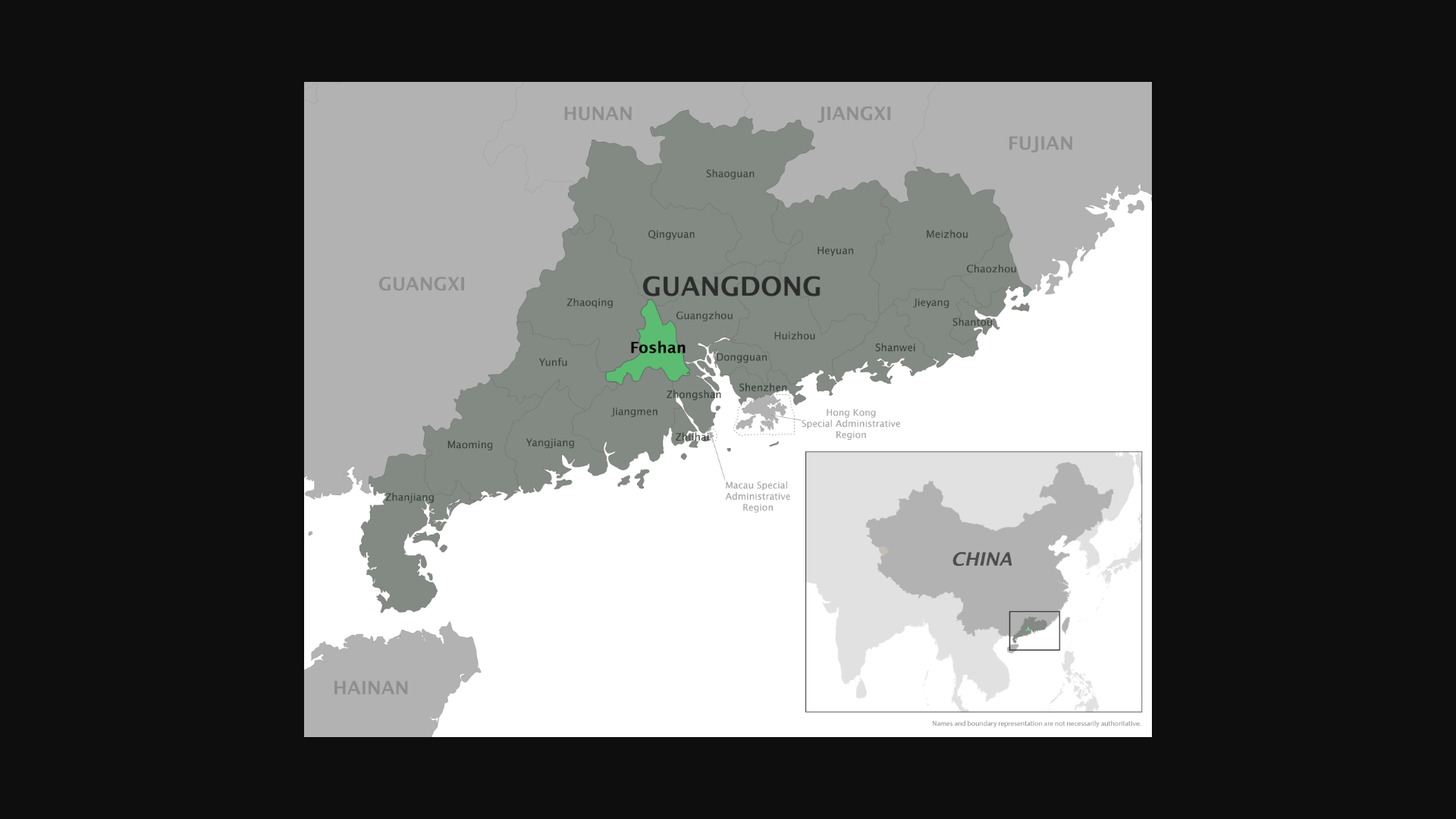
China's CDC reported its first imported case of CF in 2008. Between 2010 and 2019, local outbreaks resulting from imported instances were documented in Guangdong, Zhejiang, and Yunnan provinces.
During the summer of 2025, Foshan City, Guangdong Province, reported a cluster of CF cases.
In addition to avoiding mosquito bites, the CDC recommends vaccination as a preventive measure for Chikungunya for some travelers to countries in Asia and the Pacific Ocean.
Vaccination may be considered for individuals aged 12 years and over who are traveling to regions with a current Chikungunya outbreak.
For detailed vaccination advice, the CDC and the UK recommend that travelers schedule an appointment with their healthcare professional at least four to six weeks before departure for China.
However, even if time is short, an appointment is still worthwhile, say these health agencies.
The US Centers for Disease Control and Prevention (CDC) updated its Level 1, Practice Usual Precautions, Travel Health Advisory regarding the ongoing spread of the Oropouche virus in the Region of the Americas.
As of September 18, 2025, the CDC is reporting cases of Oropouche in Brazil, Cuba, Panama, and Peru.
For example, the Panama Ministry of Health reported that 10 human cases of locally acquired Oropouche virus disease had been detected in various districts of the Veraguas region, including Santiago, La Pena, Atalaya, and Rodrigo Luque.
Between January and the end of July 2025, about 501 cases, including one death, were reported in Panama by the Pan American Health Organization.
In the United States, states such as Florida reported 103 cases of Oropouche fever in 2024 in people who traveled to Cuba. The Florida counties reporting cases were led by Miami-Dade (61).
The CDC states that travelers to affected areas should take steps to prevent bug bites, infected midges, and mosquitoes.
This virus can also be transmitted from a pregnant woman to her fetus. Recent scientific reports have found evidence of Oropouche virus and viral RNA in patients' bodily fluids, including semen and vaginal fluids.
Currently, there are no Oropouche vaccines available for disease prevention in 2025.
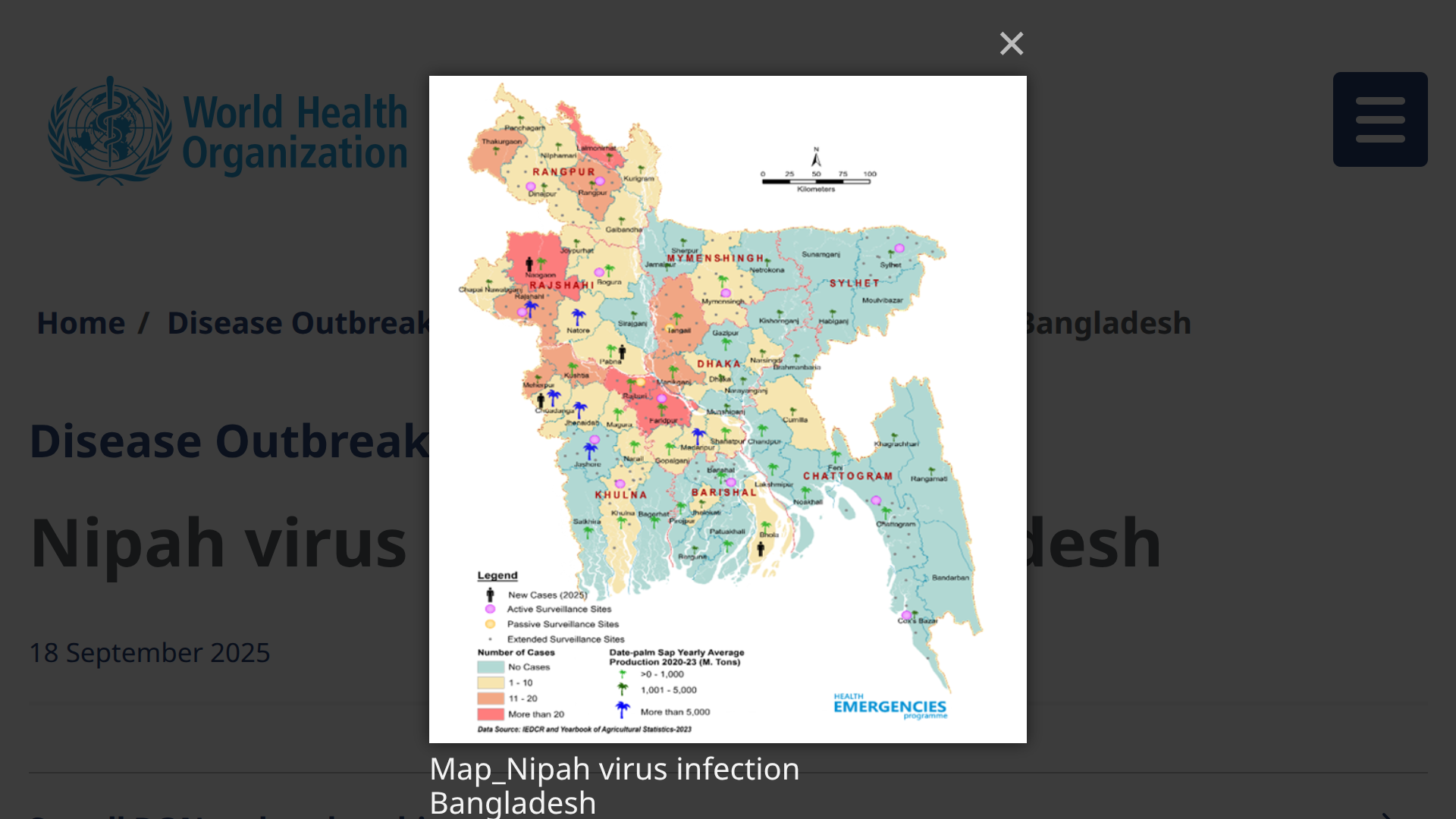
The International Health Regulations National Focal Point (IHR NFP) for Bangladesh recently notified the World Health Organization (WHO) of four confirmed fatal cases of Nipah virus (NiV) infection.
As of September 18, 2025, Bangladesh has documented 347 NiV cases through its Nipah surveillance system, which was established to detect and respond to outbreaks promptly, with a case fatality rate of 71.7%.
The WHO reported that between January and August 29, 2025, three geographical divisions in Bangladesh —namely, Barisal, Dhaka, and Rajshahi —reported these NiV patients.
Since the first recognized outbreak in Bangladesh in 2001, human NiV infections have been detected almost every year, says the WHO.
The Ministry of Health and Family Welfare in Bangladesh has implemented several public health measures with support from the WHO. The WHO assesses the overall public health risk posed by NiV at the national and regional levels to be moderate; the risk of international disease spread is considered low.
Human NiV infection is an epidemic-prone disease that can cause severe disease in humans and animals, with a high mortality rate, and outbreaks primarily occur in South and South-East Asia.
Recently, in India, NiV-related fatalities were reported.
As of August 6, 2025, Kerala State health officials have reported four cases to the WHO since mid-May, two of which have been fatal.
Since 2018, Kerala has experienced nine outbreaks of the Nipah virus, which is part of a pattern of recurring spillovers.
The WHO states there are currently no specific drugs or vaccines for NiV infection; intensive supportive care is recommended to treat severe respiratory and neurologic complications.
In 2023, the Coalition for Epidemic Preparedness Innovations invested $$100 million in four Nipah vaccine candidates.
Recently, the U.S. government announced a project to support the development of a Nipah monoclonal antibody, which is currently undergoing Phase 1 clinical trial testing in India and Bangladesh.