Search API
The Victorian Department of Health recently announced that Australia's surveillance program detected vaccine-derived poliovirus type 2 (VDPV2) in pre-treated sewage from the Western Treatment Plant in Melbourne.
Melbourne has over 5 million residents and is the host city of the Australian Open 2025, which hundreds of thousands of tennis fans attend.
The poliovirus detection (Advisory number: 241224) on December 2, 2024, is likely linked to an individual who received a live polio vaccine and shed the virus in Victoria. Victoria's Chief Health Officer, Dr. Clare Looker, wrote on December 23, 2024, that Australia remains polio-free, as this wastewater detection is not a wild-type poliovirus case.
However, the U.S. CDC says the detection of poliovirus in wastewater cannot be used to determine the total number of infected persons in a community or the portion of the infected population. The minimum number of infected individuals that can be reliably detected through wastewater testing is not known.
To alert the international community, the World Health Organization (WHO) confirmed in December 2024 that the spread of the poliovirus remained a Public Health Emergency of International Concern. In 2022, the United States was added to the WHO's list of polio-identified countries.
Over the past few years, more than 1 billion 'triple-locked' nOPV2 vaccines have been administered to prevent virus mutations.
In the United States, the inactivated polio vaccine is offered.
"Most travelers to Australia don't need many travel vaccines, but with poliovirus detected in sewage in Melbourne, a once-in-a-lifetime booster dose of polio vaccine would be prudent before departure," commented Beverly Schaefer, travel vaccine expert at Katterman's Sand Point Pharmacy, Seattle, WA.
The U.S. CDC suggests that international travelers speak with a travel vaccine expert about Japanese encephalitis, which is mainly a concern in the Murray River and the Outer Torres Strait Islands area. All international travelers should also be vaccinated against measles with the MMR vaccine.

The World Health Organization (WHO) today published an updated Disease Outbreak News regarding an undiagnosed disease in the Democratic Republic of the Congo (DRC), which was published on December 8, 2024.
As of December 27, 2024, the WHO identified this condition as an acute respiratory infection complicated by malaria.
Recent laboratory results from 430 samples indicated positive results for malaria and several common respiratory viruses, including Influenza A (H1N1, pdm09), rhinoviruses, human coronaviruses, parainfluenza viruses, and human adenoviruses.
These findings suggest a combination of common and seasonal viral respiratory infections and falciparum malaria, compounded by acute malnutrition, which has led to a rise in severe infections and deaths, disproportionately affecting young children.
This led to a significant increase in reported cases, with 891 cases documented as of December 16. However, the number of deaths reported weekly (48 deaths during this period) has remained relatively stable.
The DRC's health ministry had reported a fatality rate of 6.2%. Young children represent 64.3% of all reported cases.
The WHO wrote that this event highlights the significant burden of common infectious diseases, such as acute respiratory infections and malaria, particularly in vulnerable populations.
Additionally, the WHO recommends the programmatic use of malaria vaccines for children living in malaria-endemic areas.
The WHO and the European Medicines Agency recommend Mosquirix™ (RTS,S/AS01) and R21 / Matrix-M™ vaccines for travelers visiting malaria-endemic countries.
As of December 27, 2024, seventeen African countries are deploying malaria vaccines unavailable in the U.S.

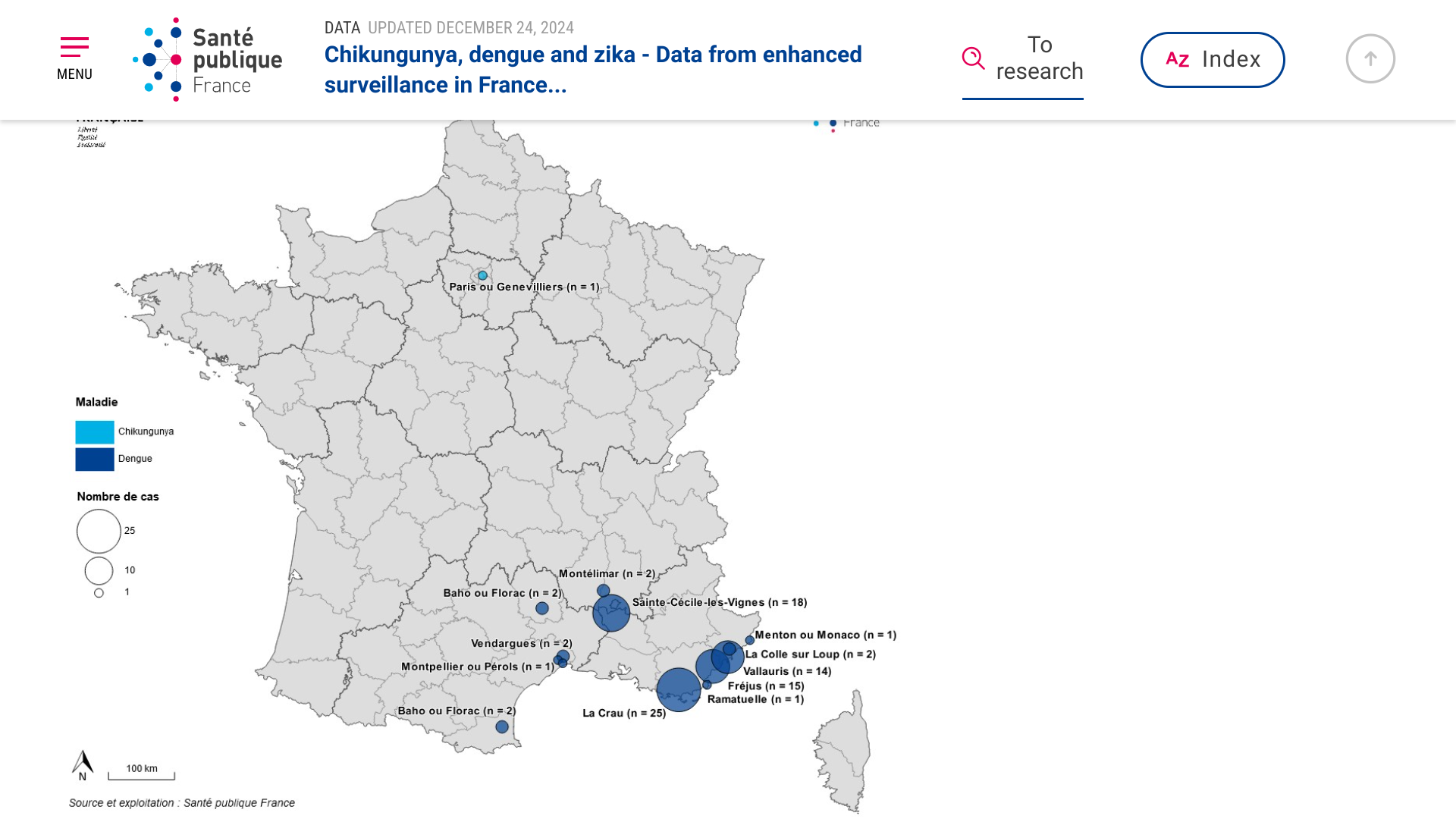
According to Regional Health Agencies (ARS), France has reported the highest number of indigenous (local) dengue cases in 2024 since the implementation of enhanced surveillance in 2006.
As of December 24, 2024, the ARS assessment for this 2024 season shows 11 outbreaks of local dengue transmission totaling 83 cases, mainly occurring in Provence-Alpes-Côte d'Azur (Marseille, 1.9 million pop.) and Occitanie.
As of December 17, 2024, 4,694 imported dengue cases had been reported this year, the highest annual number ever. Epidemiological investigations identified imported dengue cases from travelers returning to France from Guyana, Reunion, and Indonesia.
ARS also reported one Indigenous case of chikungunya for the first time in Ile de France.
Entomological and epidemiological investigations were immediately implemented for each disease outbreak, accompanied by vector control actions.
From a disease prevention perspective, France offers various chikunguna and dengue vaccines.
For travelers departing from the U.S., Valneva SE's IXCHIQ® chikungunya vaccine is offered at numerous travel clinics and pharmacies.
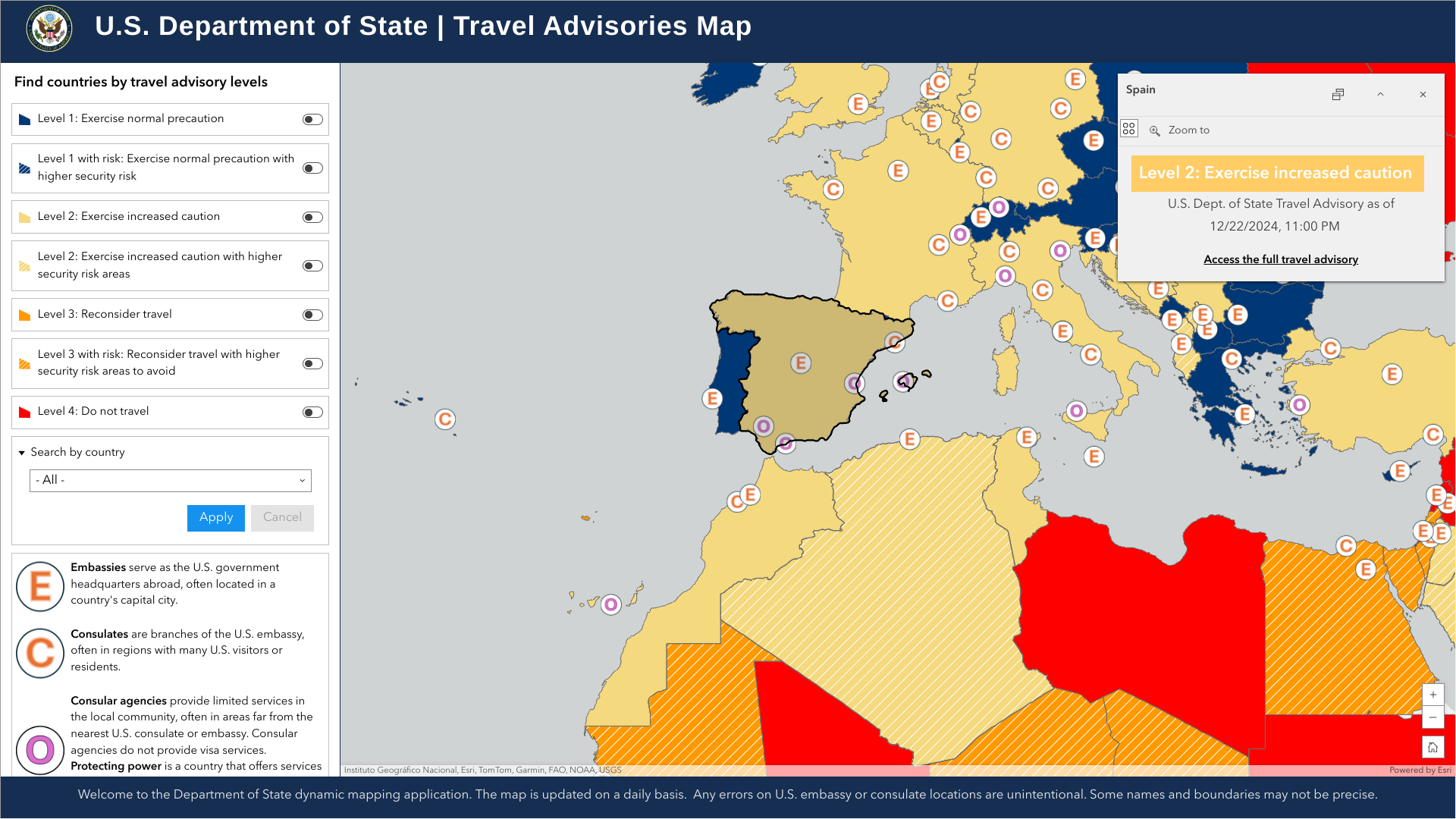
The U.S. Department of State recently reaffirmed its Level 2: Exercise Increased Caution for the Kingdom of Spain.
On December 23, 2024, the State Department stated visitors to Spain should exercise increased due to civil unrest. Furthermore, demonstrations are common and may occur in response to political or economic issues, on politically significant holidays, and during international events.
International travelers visiting Spain should enroll in the Smart Traveler Enrollment Program to receive digital alerts, which makes locating you in an emergency easier.
When in Spain, U.S. citizens can visit the U.S. Embassy at Calle Serrano, 75, 28006 Madrid.
From a health perspective, the U.S. CDC and the UK say visitors should check the list of vaccines and medicines needed at least a month before visiting Spain. For example, the ECDC reported locally acquired dengue cases in Spain in 2024.
Dengue is an Aedes-borne disease established in Spain's Catalonia region.
Colin Johnston, Senior Medical Entomologist at UKHSA, informed local media, "The increasing number of dengue (8) and malaria cases that we are seeing now in the UK are travel-related."
As of December 25, 2024, Dengue is a vaccine-preventable disease.
The Pan American Health Organization (PAHO) recently confirmed that influenza-like illnesses have increased in the North American subregion. Seasonal influenza (flu) became widespread in many sections of the United States in late December 2024.
According to the PAHO and numerical health agencies, most eligible people should get a flu shot that protects against the viruses causing infection.
The PAHO says there are four types of influenza viruses: A, B, C, and D.
As of December 25, 2024, various U.S. FDA-approved flu shots are available at local pharmacies, helping prevent severe influenza infections caused by these viruses.
The good news is pharmaceutical companies are developing vaccines that provide broad-spectrum protection against these every-mutating viruses.
A study published on December 11, 2024, in the journal MDPI, reported in a Phase 2a, double-blind, placebo-controlled study, OVX836, a nucleoprotein (NP)-based candidate vaccine, previously showed a good safety profile, a robust immune response (both humoral and cellular) and a preliminary signal of protection of 84% against confirmed symptomatic influenza after a single intramuscular dose of 180 µg, 300 µg or 480 µg.
Furthermore, T-cell responses were highly cross-reactive against various influenza A strains, both seasonal and highly pathogenic avian strains.
Last month, Osivax announced its ongoing efforts to prepare this vaccine candidate for marketing.
On November 11, 2024, the first participant was vaccinated in a Phase 2a clinical trial (NCT06582277) evaluating OVX836 as a booster in participants vaccinated three to five years ago in earlier Osivax vaccine studies.
The topline results from this trial are expected by the end of 2025.
“This milestone is a significant step forward in our mission to develop a truly broad-spectrum, lasting flu vaccine capable of addressing the ever-evolving threat of influenza. By studying the effects of a booster dose, we aim to deepen our understanding of OVX836’s potential to provide robust and sustained immune protection,” said Dr. Nicola Groth, CMO of Osivax, in a press release.
“Osivax is committed to leveraging innovative science to develop vaccines that protect individuals and help reduce the global healthcare burden associated with seasonal flu epidemics and potential pandemics.”
Osivax is a clinical-stage biopharmaceutical company that aims to develop a pan-respiratory virus vaccine that can prevent all strains of influenza in one shot. The company also intends to expand into other infectious disease indications through combinations and collaborations worldwide.




