Search API
The reemergence of yellow fever in the Brazilian state of São Paulo over the past 23 years has highlighted the need to be fully immunized before visiting endemic areas in 2025.
The São Paulo State Health Department recently confirmed the first human yellow fever case in January 2025.
According to a study published by Rev Bras Epidemiol in December 2024, five yellow fever outbreaks from 2000 to 2023 led to 679 human cases. Epizootic surveillance actions in non-human primates intensified in 2017 when the virus circulated in areas without vaccine recommendations in the state.
A previous study found the metropolitan region of São Paulo city YF outbreak during 2017–2018 revealed that 36 deaths were due to three genetic variants of sylvatic YFV that belong to the South American I genotype and that were related to viruses previously isolated from other locations in Brazil (Minas Gerais, Espírito Santo, Bahia, and Rio de Janeiro states).
According to these researchers, each variant represented an independent YF virus introduction into Sao Paulo.
"The recently confirmed case of yellow fever infection in São Paulo reinforces the importance of vaccination before traveling to many parts of Brazil, including popular urban destinations," commented Jeri Beales, MSN, RN.
"The 2017 yellow fever outbreak in Brazil marked an important change in CDC yellow fever recommendation for Brazil-bound travelers, including major cities like São Paulo, Rio de Janeiro, Curibita, and Salvador."
"Ideally, the yellow fever vaccine is given at least 10 days before arrival to a risk area, and only clinics certified with CDC can provide the vaccine," added Beales, who leads Destination Health Clinic, a Boston-area travel health provider specializing in health education and vaccination for international travelers.
As of January 21, 2025, the U.S. CDC and the U.K. Health Security Agency recommends international travelers visit a travel clinic or pharmacy to discuss travel vaccine options about one month before visiting Brazil in 2025. This year, about 8 million people may visit Sao Paulo.
According to the CDC, the yellow fever vaccine is recommended for many destinations in Brazil, including Sao Paulo, but may not required for entry.
Note: This Vax-Before-Travel news article was updated with related insight on Jan. 22, 2025.

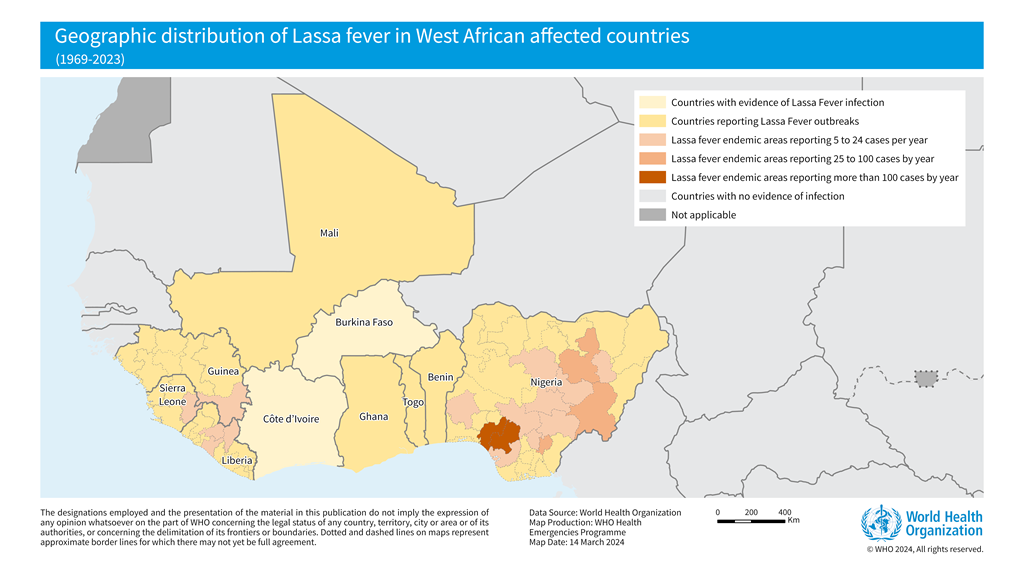
The World Health Organization (WHO) recently confirmed that it closely supports Lassa fever-endemic countries in West Africa, such as Benin, Ghana, Guinea, Liberia, Mali, Nigeria, and Sierra Leone.
As of January 19, 2025, another Lassa fever outbreak, a rare, often fatal, viral hemorrhagic fever, was confirmed in the Federal Republic of Nigeria.
The Nigeria Centre for Disease Control and Prevention (NCDC) confirmed 54 cases of Lassa fever in Ondo, Edo, and Bauchi from December 30, 2024, to January 5, 2025. The NCDC also reported 10 related fatalities, resulting in a Case Fatality Rate of 18.5%.
The NCDC continues to address the ongoing Lassa fever outbreak, which coincides with the peak season. Lassa virus was first identified in 1969 in Nigeria.
In 2024, Nigeria recorded over 1,187 confirmed cases across 28 states.
In December 2024, Dr. Jide Idris, Director-General of the NCDC, announced that the Emergency Operations Centre had been activated for Lassa fever, and the risk assessment was classified as high.
In the United States, the Iowa Department of Health and Human Services confirmed a resident died from Lassa fever in October 2024. There have been eight travel-associated cases of Lassa fever in the U.S. in the past 55 years.
As of January 21, 2025, Lassa fever vaccine candidates have not been approved for human use.
In 2024, many European countries detected poliovirus in wastewater systems, signaling the once-eradicated disease's potential resurgence.
According to the Global Polio Eradication Initiative (GPEI), poliovirus continues to be detected in Bonn, Nordrhein-Westfalen, Sachsen, and Bayern, Germany. As of January 15, 2025, eleven circulating vaccine-derived poliovirus type 2-positive environmental samples were collected in November and December 2024.
This pathogen is not the wild poliovirus type but originates from the oral polio vaccine, which contains weakened but live polioviruses. The weakened vaccine viruses can be excreted and spread by vaccinated people.
Germany's last case of wild poliovirus was recorded in 1990.
The GPEI has confirmed that various countries have also reported cases of wild polio, vaccine-derived poliovirus type 2, and circulating vaccine-derived poliovirus type 1.
On January 15, 2025, the U.S. Centers for Disease Control and Prevention (CDC) identified 39 countries at-risk for polio. This CDC list does not include Germany or the United Kingdom.
Last year, Dr Hans Henri P. Kluge, WHO Regional Director for Europe, commented in a press release, “For over 20 years, sustained efforts to achieve high vaccination coverage, quality surveillance, and rapid outbreak response have prevented the virus from re-establishing in this Region. These efforts must be commended, but also intensified as challenges to our collective defense against this virus increase.”
The CDC and the WHO recommend that all travelers to polio-affected areas in 2025 be fully vaccinated, and some people may qualify for a polio vaccine booster dose.

Moderna, Inc. recently announced ongoing support from the U.S. Department of Health and Human Services (HHS) to accelerate the development of mRNA-based pandemic influenza vaccines.
In 2023, Moderna initiated a Phase 1/2 clinical study to generate safety and immunogenicity data for an investigational pandemic influenza vaccine (mRNA-1018). The study included vaccine candidates against H5 and H7 avian influenza viruses.
Announced on January 17, 2025, the $590 million award was made through the Rapid Response Partnership Vehicle Consortium with funding from the Biomedical Advanced Research and Development Authority.
The project will support the late-stage development and licensure of pre-pandemic mRNA-based vaccines. The HHS-Moderna agreement will also expand clinical studies for up to five additional subtypes of pandemic influenza.
As of January 21, 2025, the Phase 1/2 results have not been released; however, Moderna is preparing to advance mRNA-1018 into Phase 3 clinical study.
Today's funding follows the $176 million the U.S. government awarded Moderna in July 2024.
The U.S. and European governments have invested in developing avian and pandemic influenza vaccines for years, and the U.S. Has previously approved one vaccine.
The UK government says pandemic influenza viruses are characterized by their tendency to change rapidly, their ability to spread quickly, and the routes of transmission, which contribute to the difficulty in containing an infectious global outbreak.
Influenza A viruses are most likely to cause influenza pandemics due to an extensive reservoir of these viruses in animal populations, particularly avian and swine, to which humans have no immunity.
Compared to seasonal influenza, population immunity to the new influenza A virus is nonexistent or sufficiently low to facilitate rapid person-to-person transmission and increase the severity of illness among those infected. This is generally associated with higher rates of disease and death.
Furthermore, annual 'flu=shots' are not expected to protect people from pandemic influenza viruses.
On December 11, 2024, the U.S. administration informed the media that there are no active plans to authorize the distribution of avian influenza (bird flu) vaccines.

Since 2023, the U.S. FDA has approved the Chikungunya virus vaccine, which has been deployed in various countries to curtail outbreaks, with exceptional efficacy data reported by multiple studies.
Adding to this positive trend, Valneva SE today reported further positive Phase 3 clinical trial data in adolescents for its single-shot chikungunya virus vaccine, IXCHIQ®. The vaccine showed a sustained 98.3% sero-response rate one year after a single vaccination.
These results support and strengthen the pivotal data previously reported for adolescents (12 to 17 years old), which supported filing for potential label extensions for this age group in the U.S., Europe, and Canada.
Data from this trial are also expected to support the licensure of IXCHIQ® in Brazil, which would be the first potential approval for use in Chikunguna endemic populations.
Juan Carlos Jaramillo, M.D., Valneva's Chief Medical Officer, commented, “These additional adolescent data confirm IXCHIQ®’s ability to induce a robust, long-lasting antibody response in both younger people and adults with a single vaccination."
"Given the substantial risk that chikungunya presents to individuals residing in or traveling to endemic regions, it’s imperative to ensure the vaccine is available to all age groups and has the potential to offer long-term protection, particularly in low- and middle-income countries where vaccine access is often limited."
"We are now looking forward to the first data in children, which we expect to report imminently.”
Chikungunya outbreaks have been recorded as early as 1824 in India. In 2024, over 425,00 cases and 236 related fatalities were reported in the Region of the Americas.
So far, in 2025, there have been 523 Chikungunya cases in Brazil.
IXCHIQ® is the only licensed Chikungunya vaccine available at travel clinics and pharmacies in the U.S.

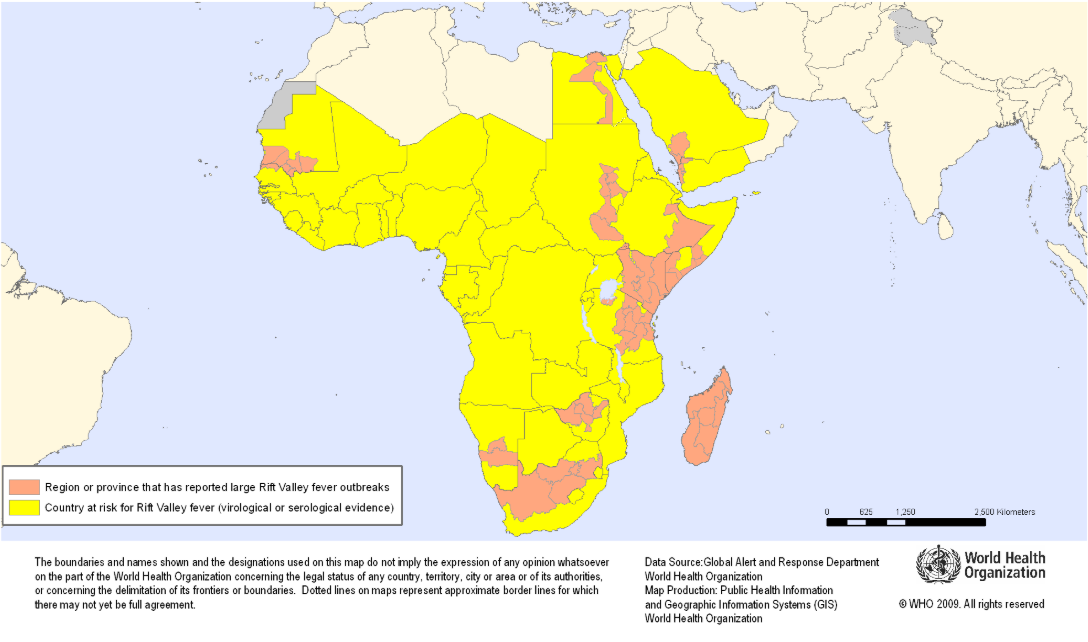
The Coalition for Epidemic Preparedness Innovations (CEPI) today announced that Afrigen Biologics aims to develop the first-ever mRNA-based vaccine against Rift Valley fever, supported by a new $6.2 million grant.
Confirmed on January 20, 2025, the researchers will work with the International Vaccine Institute to progress the new vaccine candidate through preclinical development and into Phase I clinical testing in people in either South Africa or another outbreak-affected country on the continent.
If clinical trials are successful, this vaccine could offer a critical new, locally produced tool to help combat this potentially deadly illness, which poses significant risks to human health and livestock.
Dr Richard Hatchett, CEO of CEPI, commented in a press release, “This new research will further strengthen the continent’s future preparedness and response capabilities, thereby enhancing Africa’s vaccine sovereignty and health security.”
First identified in Kenya’s Rift Valley in the 1930s, Rift Valley fever usually occurs in people following direct contact with infected animals, like sheep, goats, and cattle, or bites from infected mosquitoes, says the U.S. CDC.
The disease has also expanded in range in recent years with outbreaks in the Middle East and Indian Ocean islands, hence the need for new Rift Valley fever (RVF) vaccines.
Fortunately, a case of RVF virus spreading from person to person has never been reported.
While the majority of people infected experience mild disease, around 1-2% of those infected can develop the severe hemorrhagic form, which can cause blindness, convulsions, encephalitis, and bleeding and has mortality rates of around 50%.
Although vaccines against RVF have been registered for animals, no vaccines are available or licensed for human use. Therefore, the World Health Organization and the African Centres for Disease Control and Prevention recognize it as a priority target disease.
As of January 4, 2025, the CDC did not report any Rift Valley fever cases in 2024 or 2025.
The Houston Health Department (HHD) recently announced two measles cases associated with international travel. Both adults reside in the same Houston, Texas household and have unknown vaccination statuses.
HHD stated these are the first reported measles cases in Houston since 2018.
Texas experienced a travel-related measles outbreak in 2019, which led to 23 cases.
Measles was officially eliminated from the United States in 2000. However, as of late December 2024, 32 U.S. jurisdictions, led by Illinois and Minnesota, had reported 284 cases, many of which were travel-related.
Internationally, the U.S. Centers for Disease Control and Prevention (CDC) maintains a global Watch-Level 1 Travel Health Notice identifying measles outbreaks in 59 countries last year.
As of January 19, 2025, HHD and the CDC say the most effective way to prevent measles virus infections is to get the measles, mumps, and rubella (MMR) vaccine. Measles vaccination services are offered at most travel clinics and pharmacies.

The Centers for Disease Control and Prevention (CDC) has detected an increase in extensively drug-resistant Shigella infections in the United States over the past few years.
As of January 11, 2025, the U.S. CDC confirmed 296 Shigellosis cases have already been reported in 2025, led by New York (53) and Florida (42).
Last year, the CDC confirmed 20,621 Shigella cases nationwide, led by California (4,365) and New York (2,990).
In Northern Nevada, the Public Health (NNPH) agency identified a Shigellosis outbreak in Reno / Washoe County after a reported influx of new cases and hospitalizations. About 14 cases and nine hospitalizations were reported, although the number of cases is expected to be much higher.
However, there is a low risk of transmission to the general public in 2025.
Shigellosis is an intestinal infection that causes diarrhea, fever, and stomach pain. Shigellosis can be spread by coming into contact with the poop of an infected person, eating or drinking contaminated food or water, or through sexual contact.
According to the CDC, Shigellosis can be challenging to treat, and prevention is critical to reducing the spread of the infection.
As of January 18, 2025, the U.S. FDA has not approved a preventive vaccine. However, a tetravalent bioconjugate vaccine candidate has progressed into phase 2 clinical research.

Despite spending $4 billion annually, the number of malaria cases and deaths has not significantly changed over the past decade, especially in Africa. Last year, the WHO's African Region reported the broadest malaria outbreak burden.
Based on today's U.S. Centers for Disease Control and Prevention (CDC) Travel Health Advisory, health agencies are not optimistic about seeing any improvement in this trend by 2025.
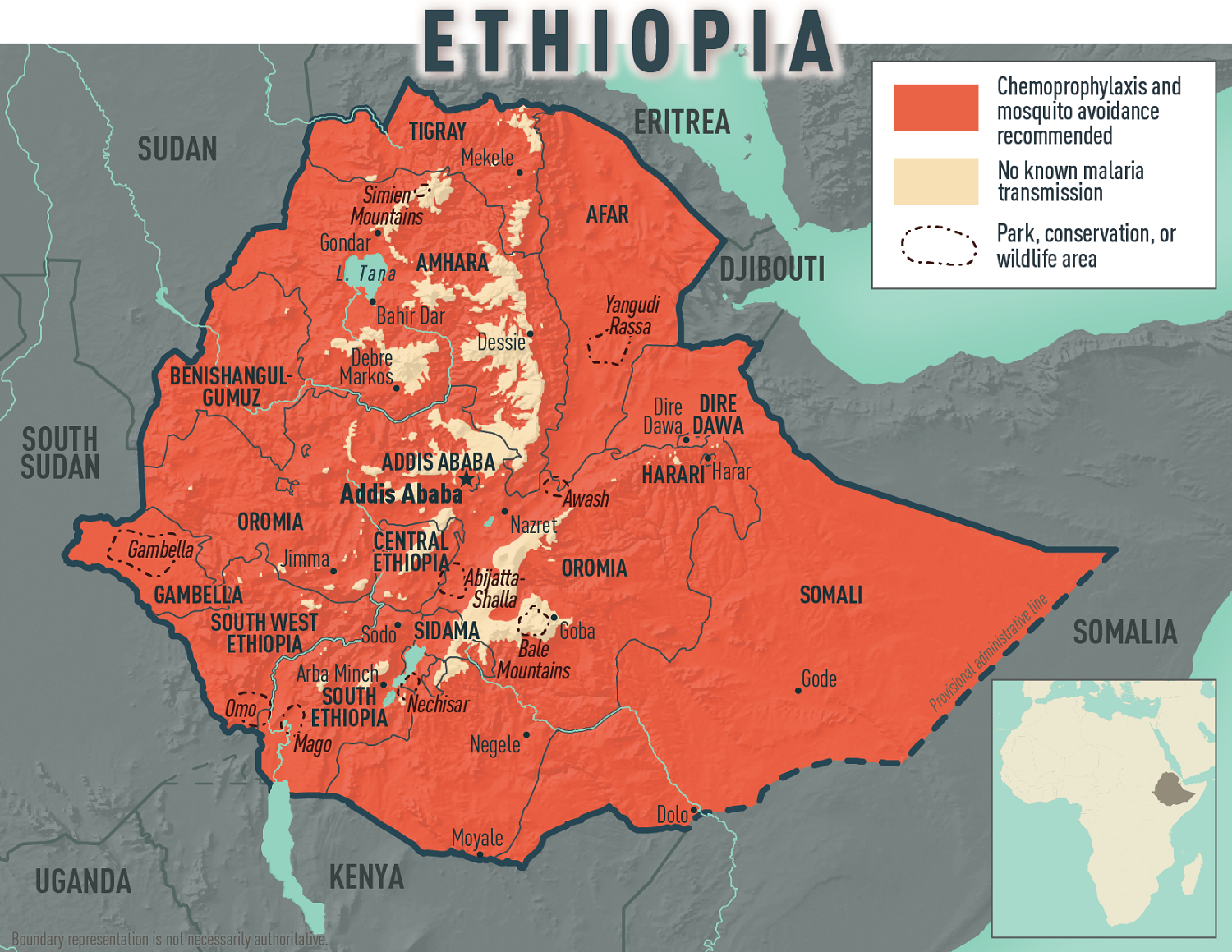
Today, the CDC confirmed an ongoing malaria outbreak in the Federal Democratic Republic of Ethiopia, affecting all 14 country regions. More than 8.4 million malaria cases were reported, the highest number of cases ever reported within a year.
To bolster Ethiopia’s fight against malaria, the United States Government, through the U.S. Agency for International Development, donated 175 computer terminals to the Ethiopian Public Health Institute on January 15, 2025.
Additional U.S. support includes over $27.5 million of antiretroviral supplies, early infant diagnostics, quality assessment panels, lab equipment, and more.
Malaria is a disease caused by a parasite that spreads to humans through the bite of infected mosquitoes, commonly found in Africa.
If you plan to travel to Ethiopia in 2025, the CDC recommends speaking with a travel health expert about which antimalarial drug is best for you. And seek medical care immediately if you develop fever, chills, sweats, headache, vomiting, or body aches during or after travel to Ethiopia.
In 2024, numerous international travelers brought malaria back with them.
As of the week ending November 23, 2024, the CDC confirmed 1,772 malaria cases, mostly among international travelers arriving in New York City (232), Texas, Miami, Florida, and Los Angeles, California.
While malaria vaccines are available in Africa, they are not FDA-approved in the U.S. and remain unavailable in the U.S.
Furthermore, innovative vaccine candidates, such as the RH5.1/Matrix-M malaria vaccine, are proceeding in late-stage clinical trials. Developed at the University of Oxford, this vaccine targets blood-stage malaria, unlike previously approved vaccines that target the pre-erythrocyte stage.