Search API
The UK Health Security Agency (UKHSA) has confirmed another Clade 1b mpox case in England, the United Kingdom's ninth since late 2024.
Previous mpox cases were confirmed in the great London area.
Beginning in 2024, clade one mpox cases were reported from countries beyond the Central African Region.
On February 4, 2025, the UKHSA reported this mpox patient had a history of travel to Uganda, a hot spot for mpox outbreaks.
Mpox is an infectious disease caused by virus infection. It was first discovered in 1958 during outbreaks of a pox-like disease. The first human case of clade one was recorded in 1970 in the Democratic Republic of the Congo.
In May 2022, a global outbreak of clade two mpox was confirmed.
Mpox is a vaccine-preventable disease, with approved vaccines available in the UK, the United States, and various other countries in 2025.
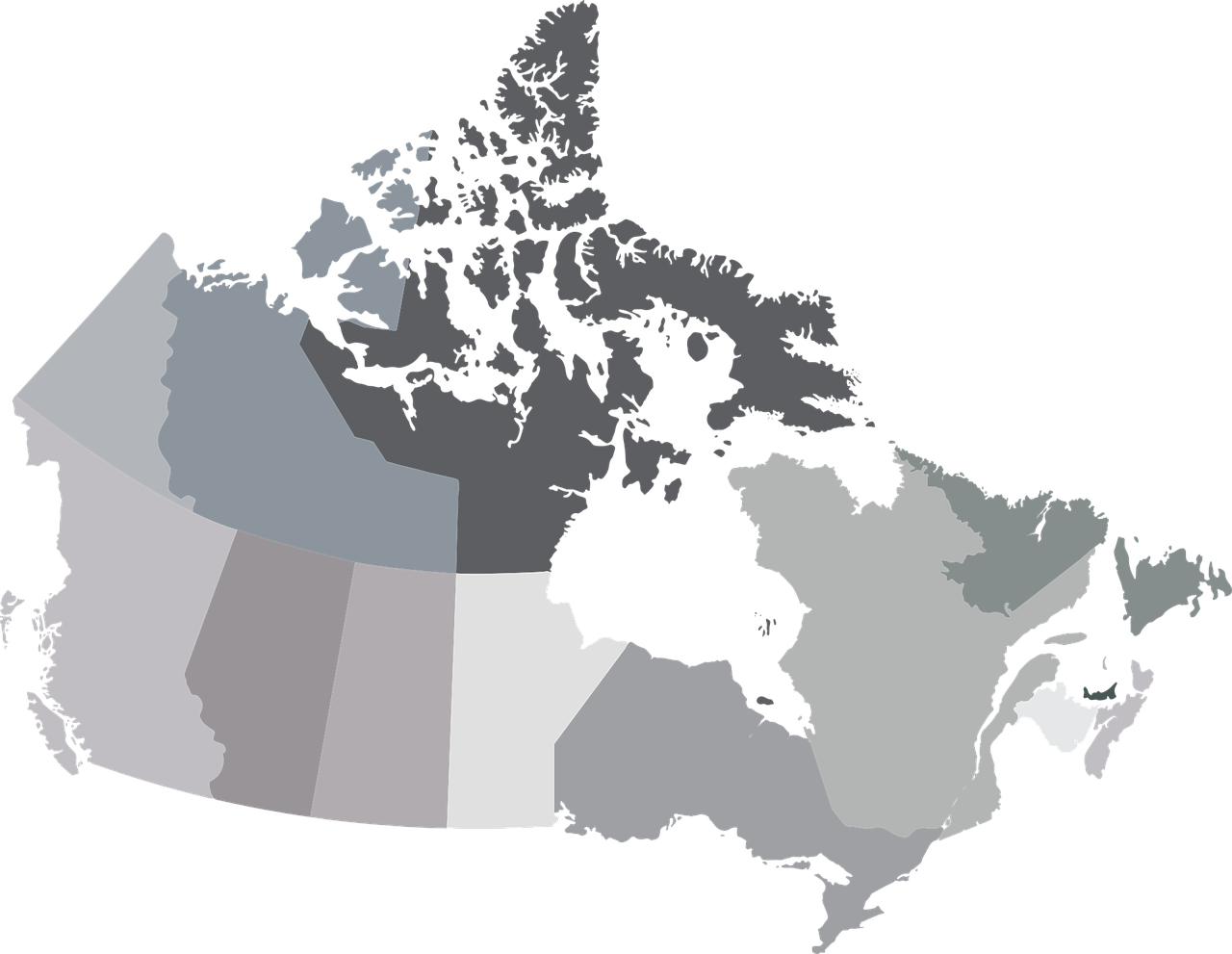
Various Canadian public health officials are reporting excessive measles outbreaks in 2025. This is essential news as Canada's provinces are preferred destinations for international travelers.
As of February 5, 2025, 81 cases (54 confirmed and 27 probable) of measles had been reported in Ontario in 2024 and 2025.
Quebec is facing its second measles outbreak since 2024, with 16 confirmed cases in Laurentides, Montréal, and Laval.
And Manitoba public health officials recently confirmed five measles cases in 2025.
In 2024, 141 measles cases and one congenital rubella were reported in Canada.
Since most of these measles cases are related to unvaccinated people, Canadians are encouraged to consult travel health notices for information on areas where measles is circulating.
The European Centre for Disease Prevention and Control reported measles cases in 30 countries last year.
Seperately, the U.S. CDC identified 59 countries reporting measles cases and issued a Travel Health Advisory. Measles vaccination services are generally offered at clinics and pharmacies in the United States.
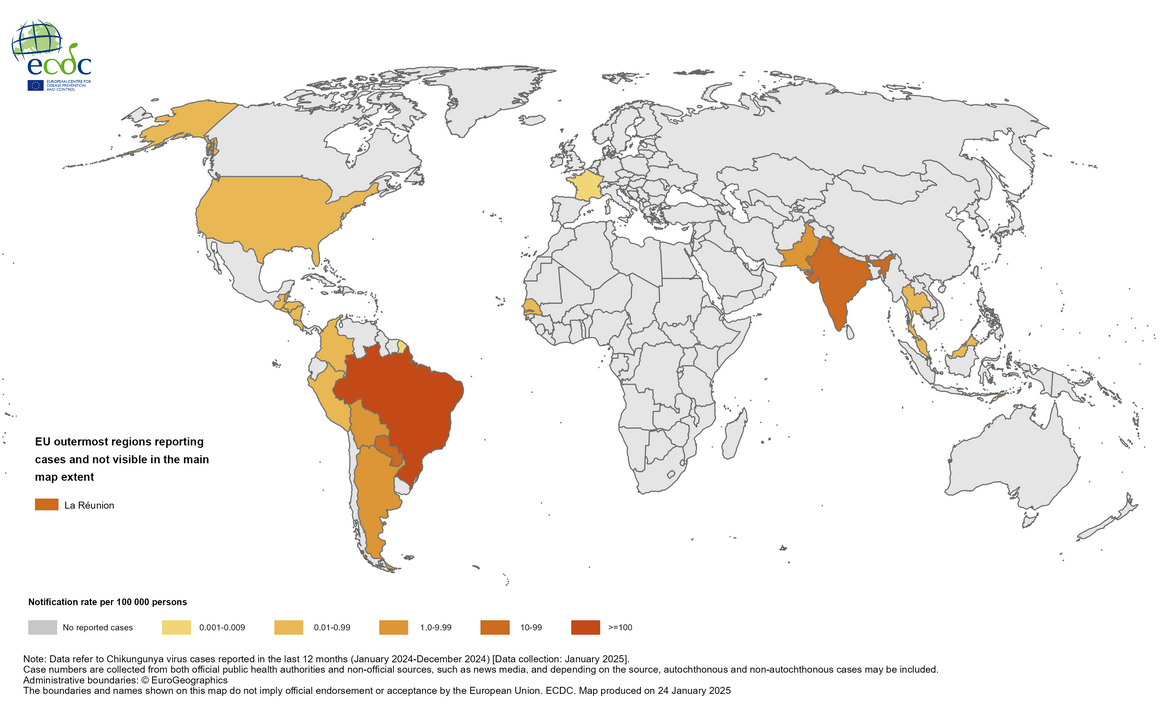
The European Centre for Disease Prevention and Control (ECDC) reported that since the beginning of 2025, and as of late January, over 5,000 Chikungunya virus disease (CHIKVD) cases and two related fatalities have been reported in three countries in the Region of the Americas (Brazil, Paraguay, and Colombia) and one in Europe.
While the ECDC has not had any autochthonous (local) cases of CHIKVD in mainland Europe in 2025, 138 cases have been reported from the French overseas department of La Réunion, located off the east coast of Africa.
In 2024, about 620,000 CHIKVD cases and 213 related fatalities were detected in countries in the Americas (15), Asia (6), Africa (1), and Europe (1).
As of February 5, 2025, one Chikungunya vaccine has been approved in Europe, the United States, and the United Kingdom. Valneva SE's IXCHIQ® Chikungunya vaccine is recommended for most travelers visiting outbreak areas.
The U.K. Medicines and Healthcare products Regulatory Agency (MHRA) announced today that it has approved Valneva SE's IXCHIQ® vaccine chikungunya vaccine (live) to protect adults against chikungunya disease.
On February 4, 2025, Julian Beach, MHRA Interim Executive Director of Healthcare Quality and Access, released a press release stating, "Patient safety is our top priority, which is why I am pleased to confirm the approval of the first vaccine in the UK to protect adults 18 years and older against Chikungunya disease."
This approval is essential as about 900,000 U.K. travelers visit India annually, and over five years, India recorded the second-highest number of chikungunya cases worldwide.
Dr. Richard Hatchett, CEO of the Coalition for Epidemic Preparedness Innovations (CEPI), commented in a press release on February 5, 2025, "Today's MHRA approval of Valneva's IXCHIQ® vaccine is an important step forward in protecting UK citizens traveling to affected countries—but the fight is not over."
"Our work now focuses on expanding access to vaccine doses, at an affordable price, in those endemic regions."
"As a major investor in CEPI, the UK Government is providing vital support to advance this goal, helping to make the vaccine accessible to those in Low- and Low-income countries who are most at risk from the disease while also protecting their population."
The IXCHIQ® vaccine has already been approved in the United States, Europe, and Canada.
Note: This news article was updated on Feb. 5, 2025, to include CEPI's quote.

A research study was recently conducted to assess the safety and tolerability of the tetravalent live-attenuated dengue vaccine, which is commercially known as Qdenga®. The study provides important insights into reactogenicity and may help improve vaccination strategies in dengue-naïve populations.
The study results were published in the Journal of Travel Medicine on February 2, 2025. Vaccine-related reactions were frequently reported, predominantly after the first dose in dengue-naïve participants.
While vaccine coadministration was a common strategy, it did not significantly increase side effects.
After the first dose, 51% of the participants reported systemic reactions, such as headache (40% (190/474)), weakness (40% (189/474)), and malaise (32% (154/474)), which were most pronounced between days 7 and 11 after vaccination.
After the second dose, localized signs and symptoms such as pain at the injection site (22% (n = 55/250)) were more common. Fever was more common after the first dose (20% (96/474)) vs. 2% (6/250) after the second.
A total of 334 (28%) coadministrations were reported, whereby AEs were reported in 47% (157/333) of participants, with the highest prevalence observed when combined with the Japanese encephalitis vaccine (56.8%, (42/74)).
Differences in age groups were observed, with decreased reactions in older people (≥ 65 years).
As of February 2025, Qdenga is not offered in the United States and is in limited supply globally.
Later this year, Butantan Institute's single-dose, tetravalent, live attenuated Butantan-DV dengue vaccine may become available in Brazil, where it conducts phase 3 clinical trials.
As the world reopened following the recent pandemic, international travelers sought innovative vaccines to protect themselves from infectious diseases.
For example, Bavarian Nordic A/S announced results for 2024 today, stating that its travel health business demonstrated strong growth of 22% in vaccine sales. Rabipur®/RabAvert® (rabies) and Encepur® drove this performance.
Paul Chaplin, President and CEO of Bavarian Nordic, released a press release on February 3, 2025, stating, "Our (vaccine) portfolio continues to grow, and we are truly excited to launch our chikungunya vaccine for travelers over 12 years old in Europe and the U.S. later this year."
"We are also continuing to expand our partnerships to improve access to critical vaccines for vulnerable populations around the globe."
Research reveals that about 1.4 billion air passengers traveled in 2024, a number that may increase by 9.9% annually through 2028.
Furthermore, last-minute travelers deferred about 18% of protective vaccines because of insufficient time before departure.

The Ministry of Health and Medical Services of the Republic of Fiji announced today a dengue fever outbreak in the Western Division.
As of February 3, 2025, about 200 dengue cases have been reported for the Western Division since the beginning of this year. Most cases from the Western Division belong to the 10-29 age group.
The Ministry wrote in a press release, 'More cases of dengue fever are expected during the rainy season, which lasts until April 2025.'
In 2024, over 2,033 dengue fever cases were reported in Fiji.
The U.K. Travel Health Pro advises' taking usual precautions' when visiting Fiji. However, the U.S. CDC does not list Fiji in its Global Dengue Outbreak notice.
The U.K. says dengue is a mosquito-transmitted infection caused by the dengue virus. There are four types: DENV-1, DENV-2, DENV-3, and DENV-4. A second-generation vaccine has been found effective against three types but has limited availability in 2025.
Located in the South Pacific Ocean, north-northeast of New Zealand, Fiji is an archipelago of about 100 inhabited islands. It is a tourist hot spot, welcoming over 900,000 visitors in 2024, many from Australia, New Zealand, and North America.
Should U.S. citizens need local assistance, the U.S. Department of State's embassy is located at 158 Princes Rd, Tamavua Suva, Fiji Islands.