Search API
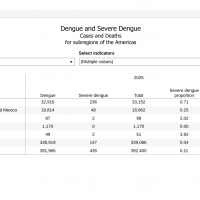
Throughout the Region of the Americas, the Federative Republic of Brazi has been the unfortunate leader in the multi-year Dengue fever outbreak.
In Brazil, the Municipal Health Department of São Paulo reported the most Dengue cases in 2024, about 2.1 million.
To reduce the number of pediatric Dengue cases in 2025, the Municipal Health Department of the capital advised parents and guardians on February 7, 2025, to take children aged 10 to 14 for Dengue vaccination, which occurs at Basic Health Units (UBSs).
UBSs also actively searched the territories in 2024 and 2025 to ensure that Dengue immunization reached this population. The government has estimated 600,000 children in Sao Paulo, and 259,000 first doses (38%) and 134,000 second doses (20%) have been administered to date.
As of February 12, 2025, Takeda's QDENGA® two-dose vaccine is available in Brazil and the Americas but not in the United States.

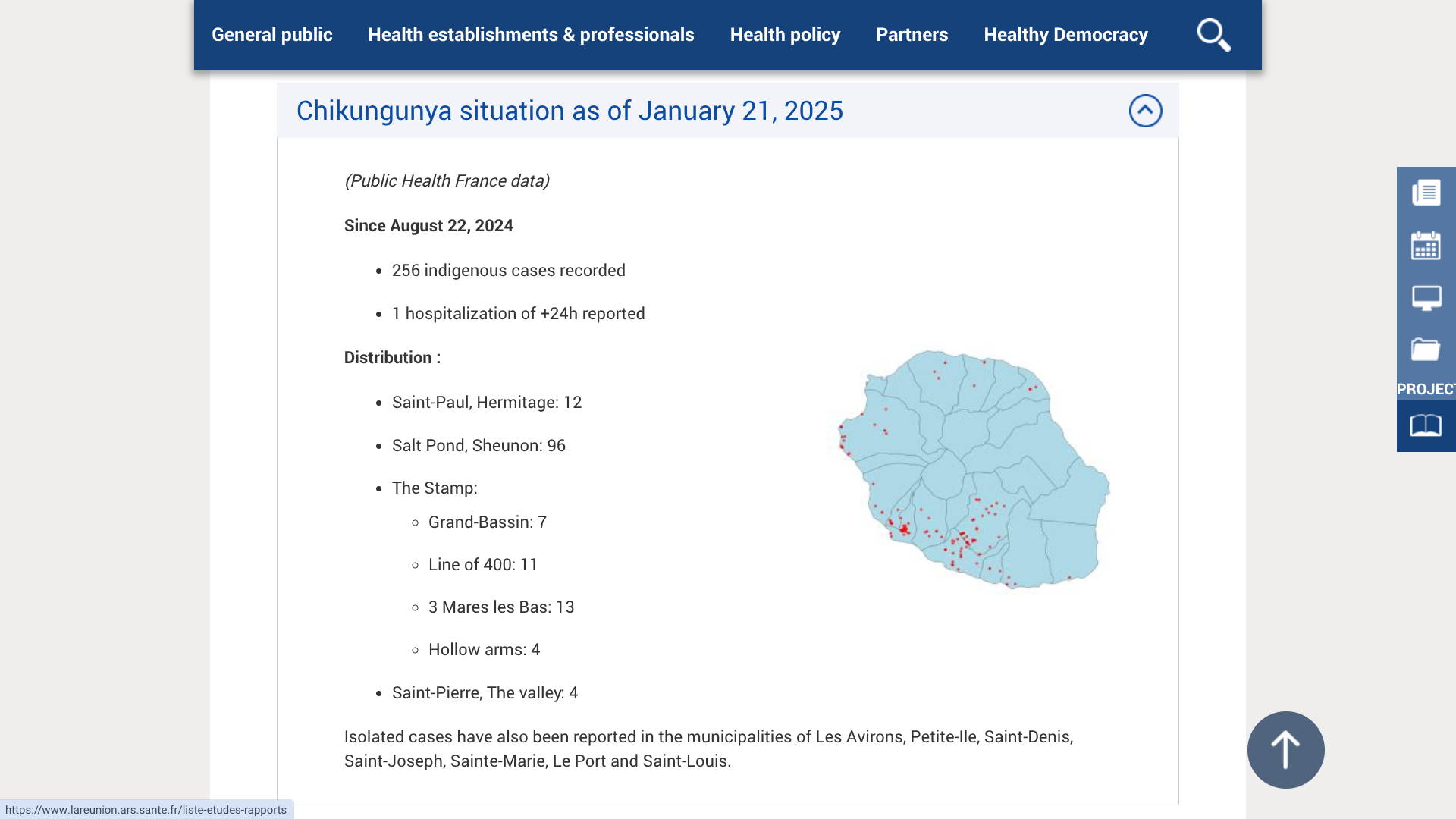
France is one of the most popular tourist destinations, reaching about 100 million guests annually. Its overseas department, La Réunion, welcomes about 500,000 vacationers to its beautiful mountains and beaches.
However, due to the increase in the number of Chikungunya virus cases and its continued infections, and on the proposal of the Director General of the ARS Gérard COTELLON, Patrice LATRON, France's Prefect of La Réunion, has triggered level 3 of the ORSEC "arboviruses" system, which corresponds to a low-intensity epidemic.
As of February 11, 2025, and since August 23, 2024, ARS Reunion has confirmed 783 indigenous cases, including 671 since the start of 2025.
The municipalities of Étang-Salé and Le Tampon still have the highest number of Chikungunya cases.
'As soon as a case of Chikungunya is reported, the ARS intervenes in the affected area, without waiting for confirmation of the case by the medical biology laboratory, to reduce the risk of spreading the virus.'
The last major Chikungunya outbreak in La Réunion was from 2005 to 2006.
The ongoing outbreak in La Réunion is caused by Ae. albopictus, the primary vector, due to the adaptation of the ECSA CHIKV genotype.
While the U.S. CDC has yet to highlight La Reunion's Chikungunya outbreak as a travel risk, it says international travelers should arrange an appointment with a travel vaccine specialist at least four to six weeks before departing for France. An appointment with a travel expert provides an opportunity to assess which vaccines are appropriate for your trip abroad in 2025.
When departing for France or La Reunion from the United States, Valneva SE's IXCHIQ® chikungunya vaccine is commercially offered by various travel clinics and pharmacies. And in 2025, this innovative vaccine can be found throughout Europe.
The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) recently published a draft agenda for its meeting scheduled for February 26-28, 2025.
This ACIP meeting will be hosted at the CDC in Atlanta, GA, is open to the public, and will be broadcast digitally on YouTube.
On Wednesday, February 26, Dr. Keipp Talbot (ACIP Chair) will welcome the new ACIP members and lead discussions on Meningococcal, Chikungunya, Influenza, Respiratory Syncytial Virus (RSV) disease, and related vaccines.
The ACIP will vote on specific recommendations, which are then sent to the CDC"s Director for consideration and/or approval.
As of January 24, 2025, Susan Monarez, PhD, became the Acting CDC Director, First Assistant to the Director, and Principal Deputy Director.
On Thursday, the 27th, the committee will continue discussing RSV vaccines and then review Human Papillomavirus, Mpox, Pneumococcal, and Lyme diseases.
Then, on Friday, the 28th, COVID-19 and Cytomegalovirus vaccines will be discussed.
The CDC has already scheduled additional ACIP meetings for June 25-26 and October 22-23, 2025.
The ACIP includes up to 19 voting members responsible for making recommendations. The Secretary of the U.S. Department of Health and Human Services selects these members. ACIP voting members are independent medical and public health experts who do not work for the CDC.
These ACIP meetings are essential as the CDC sets the U.S. adult and childhood immunization schedules based on recommendations from ACIP.
According to the Centers for Disease Control and Prevention's recent update, the gastrointestinal illness outbreak on the Royal Caribbean International ship Radiance of the Seas affected 7.4% of its passengers.
Additionally, the crew who reported being ill during the voyage was 8 of 910 (0.9%).
As of February 10, 2025, the writes that it's not clear what caused the outbreak, but said symptoms of infected passengers included vomiting and diarrhea.
The Voyage (20136) was between February 1, 2025, and February 8, 2025.
Per CDC protocol, the Vessel Sanitation Program remotely monitored the onboard situation, including reviewing the ship's outbreak response and sanitation procedures.
This new incident is the 8th in 2025.
The CDC confirmed that 2024 was the worst year for gastrointestinal illness outbreaks (18) on cruise ships in over a decade. Norovirus was the most common cause.
'Norovirus is often a cause of GI illness outbreaks on cruise ships, but we don't always know the cause of the outbreak when we begin an investigation.' writes the CDC.
The CDC says there are no U.S. FDA-approved norovirus vaccines available in 2025.

The Republic of Singapore's Ministry of Health (MOH) recently confirmed one imported case of vaccine-associated paralytic poliomyelitis in a five-month-old Indonesian who arrived in Singapore for medical treatment at the National University Hospital upon arrival.
According to the MOH's press release on February 7, 2025, the infant is immunocompromised and was previously vaccinated with one dose of oral polio vaccine (OPV) and one dose of inactivated polio vaccine (IPV).
Vaccine-associated paralytic poliomyelitis is a rare adverse event that occurs when an individual develops paralytic polio after receiving OPV. The risk is higher for immunocompromised persons, for whom IPV is recommended instead of OPV.
Many countries have progressively switched to offering IPV, and Singapore stopped using OPV in 2021.
Singapore, an island country and city-state in Southeast Asia, has not reported any locally acquired polio cases since 1978.
Singapore has maintained its polio-free status by providing high polio vaccination coverage, maintaining high environmental hygiene and sanitation standards, and establishing a surveillance system to detect possible poliomyelitis cases.
The MOH says vaccination is the most effective protection against poliomyelitis. Children receive five vaccine doses under the National Childhood Immunisation Schedule.
As of February 10, 2025, the MOH says there is a low risk of community transmission.
A recent Eurosurveillance analysis stated, 'Until global eradication is achieved and as long as poliovirus is circulating anywhere, importations into Europe are inevitable.'
In January 2025, the U.S. Centers for Disease Control and Prevention (CDC) reissued a Global Polio Alert—Level 2, Practice Enhanced Precautions Travel Health Notice, identifying polio outbreaks and virus detections in 39 countries.
The CDC has recommended routine and travel vaccinations such as yellow fever and measles before visiting Singapore in 2025.
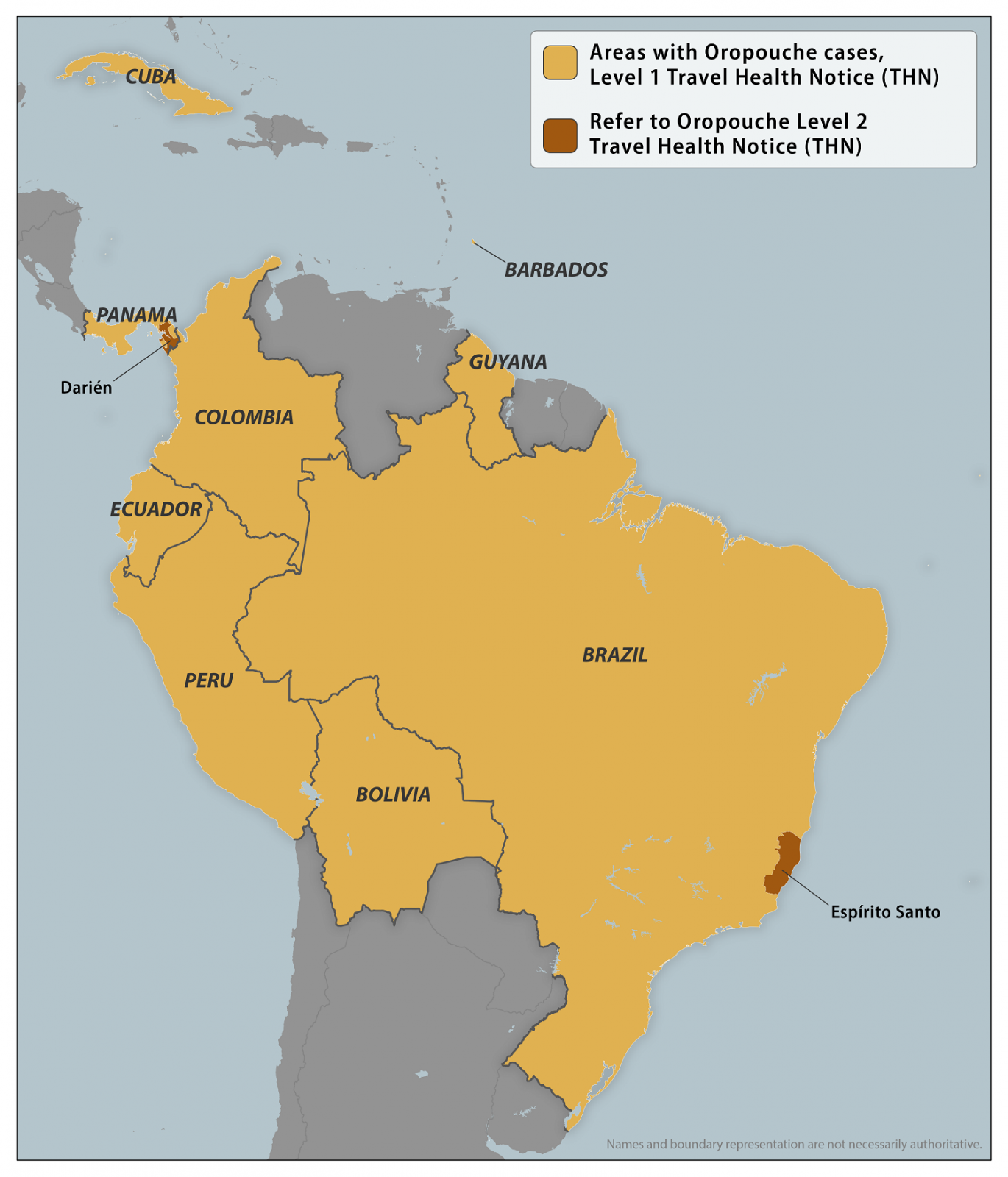
Cuba's Oropouche fever outbreak set new records in 2024 and started in 2025, heading to new highs.
On January 30, 2025, the PAHO reported Dr. José Raúl de Armas, head of the Department of Communicable Diseases at MINSAP, provided an update on the epidemiological situation in the country, which has reached 100% of Cuba's provinces.
To date, there have been 23,639 suspected cases and 626 confirmed cases. Among the latter were 76 patients with Guillain-Barré Syndrome, 25 with encephalitis, and 15 with meningoencephalitis.
Florida, Cuba's western neighbor, confirmed 103 travel-associated Oropouche fever cases in 2024.
According to the U.S. Centers for Disease Control and Prevention (CDC), the Oropouche virus is spread primarily through the bites of infected small flies and mosquitoes. Symptoms typically start 3–10 days after being bitten and last 3–6 days. Most people recover without long-term effects. There is no specific treatment for Oropouche.
To alert international travelers of this health risk, the CDC issued Travel Health Advisories for various countries in the Region of the Americas. Furthermore, the CDC confirmed there are no Oropouche vaccines available in 2025.
The U.S. Centers for Disease Control and Prevention (CDC) recently stated that 'seasonal influenza activity remains elevated and continues to increase across the country.'
As of February 7, 2025, the CDC disclosed an unfortunate trend; ten additional influenza-associated pediatric fatalities were reported last week, bringing this year's total to 57.
During the last flu season, the CDC reported 207 children died from influenza infections.
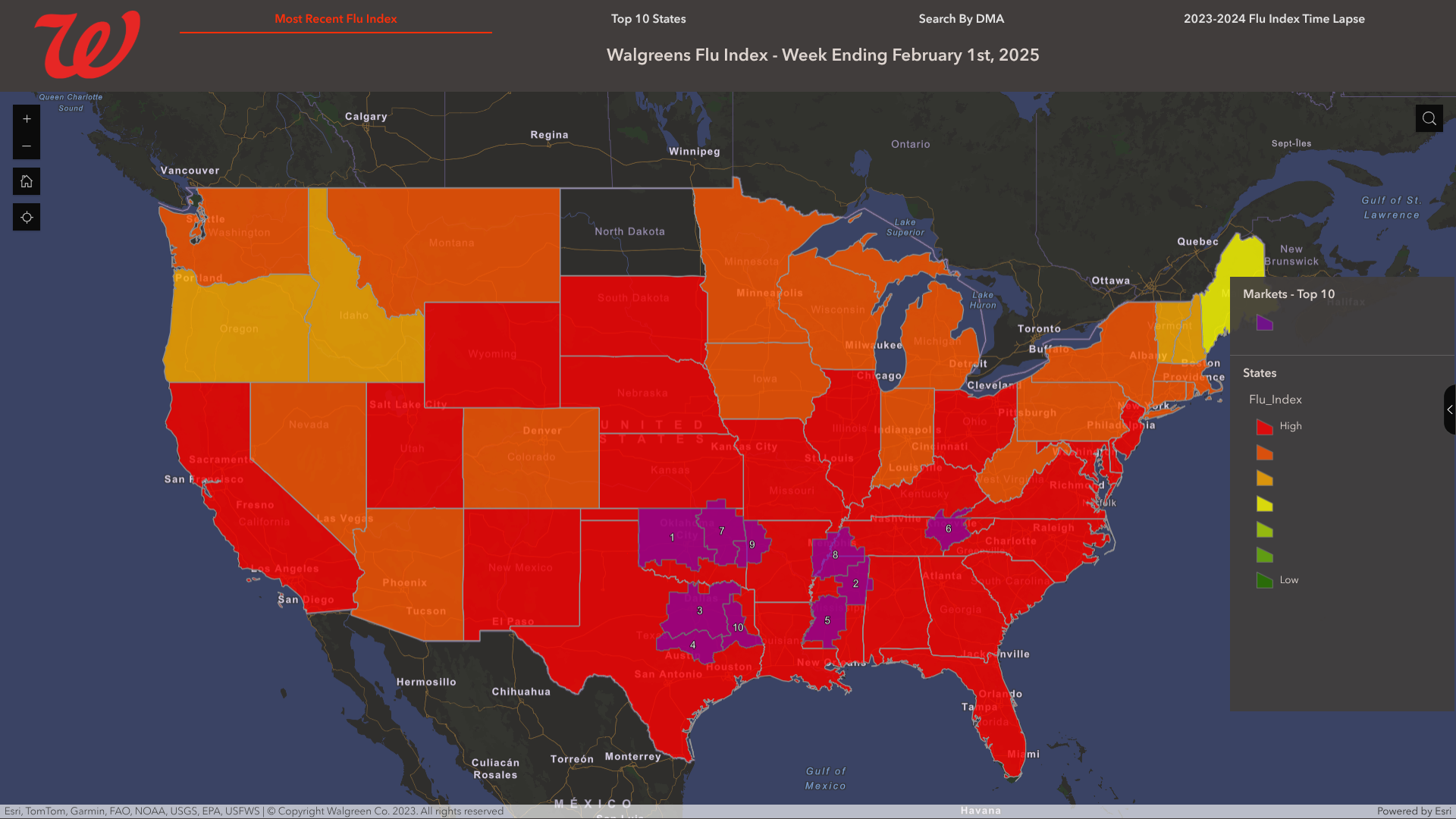
From a geographic perspective, the Walgreens Flu Index has identified its leading markets for influenza-related activity. As of February 1, 2025, the leading Designated Market Areas with flu activity were:
- Oklahoma City, Okla.
- Columbus-Tupelo-West Point-Houston, Miss.
- Dallas-Ft. Worth, Texas
- Waco-Temple-Bryan, Texas
- Jackson, Miss.
- Knoxville, Tenn.
- Tulsa, Okla.
- Memphis, Tenn.
- Ft. Smith-Fayetteville-Springdale-Rogers, Ark.
- Tyler-Longview (Lufkin & Nacogdoches), Texas
The CDC continues to encourage most people to get their annual flu shot at health clinics and pharmacies. The CDC stated that over 92 million flu shots had been distributed in the U.S., targeting the 2024-2025 flu season.
Note: The Walgreens Flu Index provides state—and market-specific information regarding flu activity. It is compiled using retail prescription data for antiviral medications used to treat influenza across Walgreens locations nationwide. The Flu Index is not intended to illustrate levels or severity of flu activity but rather to illustrate which populations are experiencing the highest incidence of flu.
Ireland's Health Protection Surveillance Centre (HSE) recently detected one imported case of clade I mpox.
As of February 5, 2025, the individual was receiving specialist care in a hospital in Dublin.
The Irish resident had returned to Ireland following travel to the Democratic Republic of the Congo, where both clades of mpox are circulating in the community.
HSE stated in a media release that 'the (clade I mpox) risk to the Irish public remains low.
Additionally, cases of clade IIb mpox in Ireland remain low, with 8 cases reported in 2025. There were 25 cases confirmed in 2024, 13 cases in 2023, and 227 cases in 2022.
To Irelands' east, the UK Health Security Agency recently confirmed England's 9th clade I case in various cities.
As of February 8, 2025, mpox vaccines are approved by various countries and are commercially available in the United States.