Search API
Novavax, Inc. today announced progress in advancing its corporate growth strategy through its partnership with Sanofi, a Paris, France based company.
Novavax has achieved a milestone associated with its Phase 2/3 clinical trial for its COVID-19 vaccine in children, triggering Sanofi's first $50 million milestone payment.
Novavax's COVID-19 vaccine is included in Sanofi's two combination vaccine candidates for the prevention of influenza and COVID-19, for which Phase 1/2 trials were initiated, and the U.S. FDA Fast Track designation was recently granted in the U.S.
n addition to milestones for the stand-alone COVID-19 vaccine, the agreement also includes combination products developed by Sanofi including Novavax's COVID-19 vaccine, which present a potential opportunity of up to an additional $350 million for Novavax in future milestones.
In addition, stand-alone COVID-19 sales and potential sales of any Sanofi combination products would net Novavax ongoing tiered royalties.
Further, Novavax is eligible to receive up to $200 million for the first four products created by Sanofi utilizing its Matrix-M adjuvant and up to $210 million in milestone payments for each product, including Matrix-M thereafter, plus ongoing royalties for all Sanofi products utilizing Matrix-M.

The Queensland Public Health and Scientific Services, Communicable Diseases Branch, recently issued a public health alert about measles outbreaks.
As of December 13, 2024, measles cases have been reported among travelers to Victoria, Western Australia, and Queensland this year.
This alert stated clinicians in Australia should be alert for signs and symptoms of measles, particularly in international travelers or those close contacts potentially exposed to measles.
To alert the global community, the U.S. CDC reissued a Level 1 - Practice Usual Precautions, Travel Health Advisory, regarding measles outbreaks in 56 countries. As of late November 2024, this CDC alert did not include Australia.
As of December 6, 2024, the U.S. CDC reported 283 measles cases in 32 jurisdictions this year.
The CDC wrote that travelers are at risk of measles if they have not been fully vaccinated at least two weeks before departure or have not had measles in the past and have traveled internationally. In the U.S., measles vaccines are generally available at travel clinics and pharmacies.
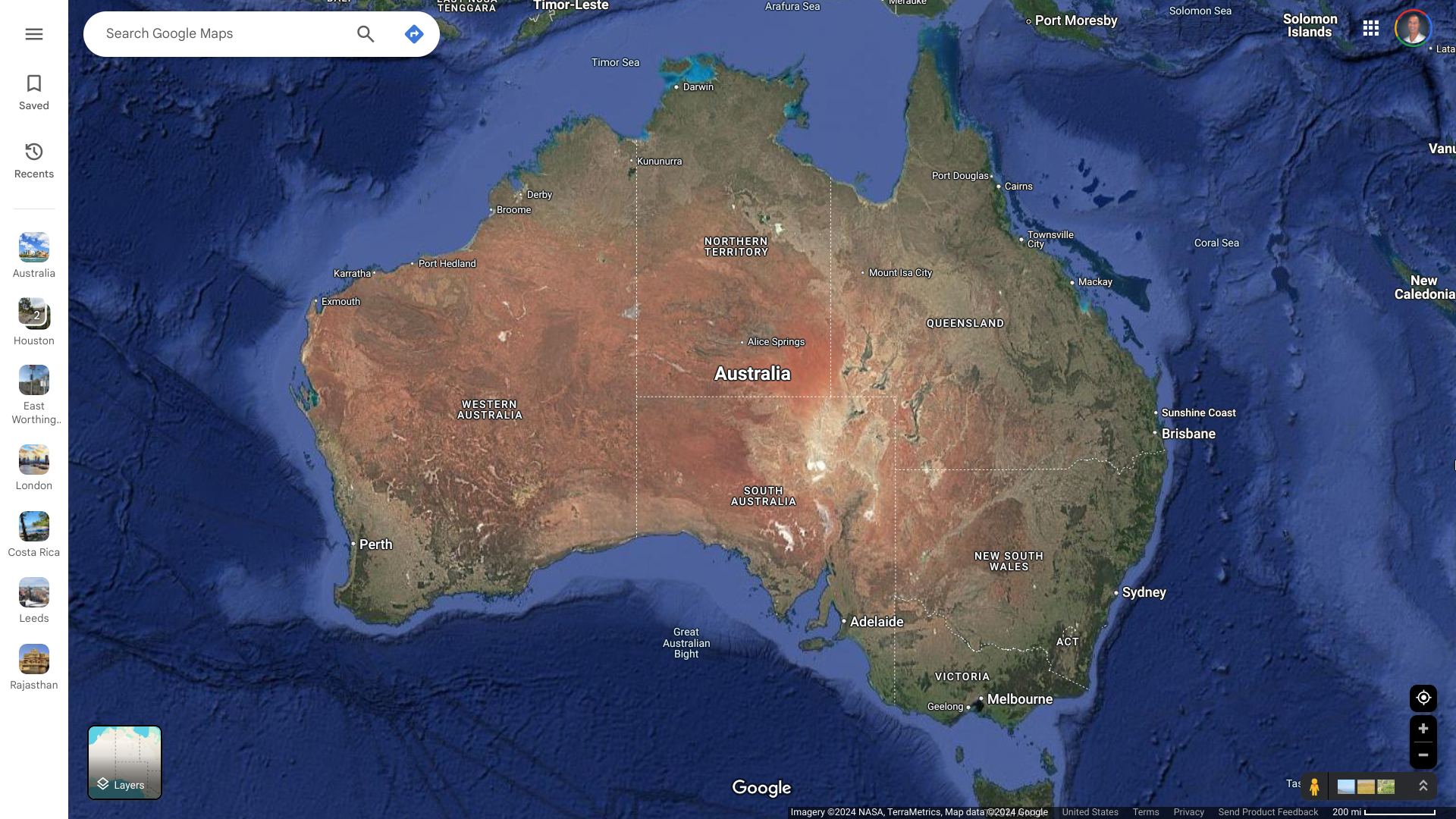
The European CDC today reported that the United Kingdom confirmed the notification of circulating vaccine-derived poliovirus type 2 (cVDPV2) isolated from environmental samples.
As of December 13, 2024, the ECDC stated that cVDPV2 was isolated from environmental samples collected in Leeds, London, and East Worthing over the past few months. However, no human cases of polio have been reported in the U.K. in 2024.
Regarding polio preventive vaccines, a recent report from the September Strategic Advisory Group of Experts on Immunization meeting recommended the concomitant use of IPV and type 2 novel oral polio (nOPV2) vaccines in initial outbreak response vaccination campaigns.
Since 2000, the IPV vaccine has been offered in the United States.
And over the past few years, about 1.1 billion nOPV2 vaccine doses have been administered in Africa.
Since August 2024, the U.S. CDC has issued a Level 2—Practice Enhanced Precautions, Travel Health Advisory, listing 37 countries reporting poliovirus detections this year. The CDC writes that adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine before traveling to any destination listed.
The U.K. is not included in the CDC's current list.
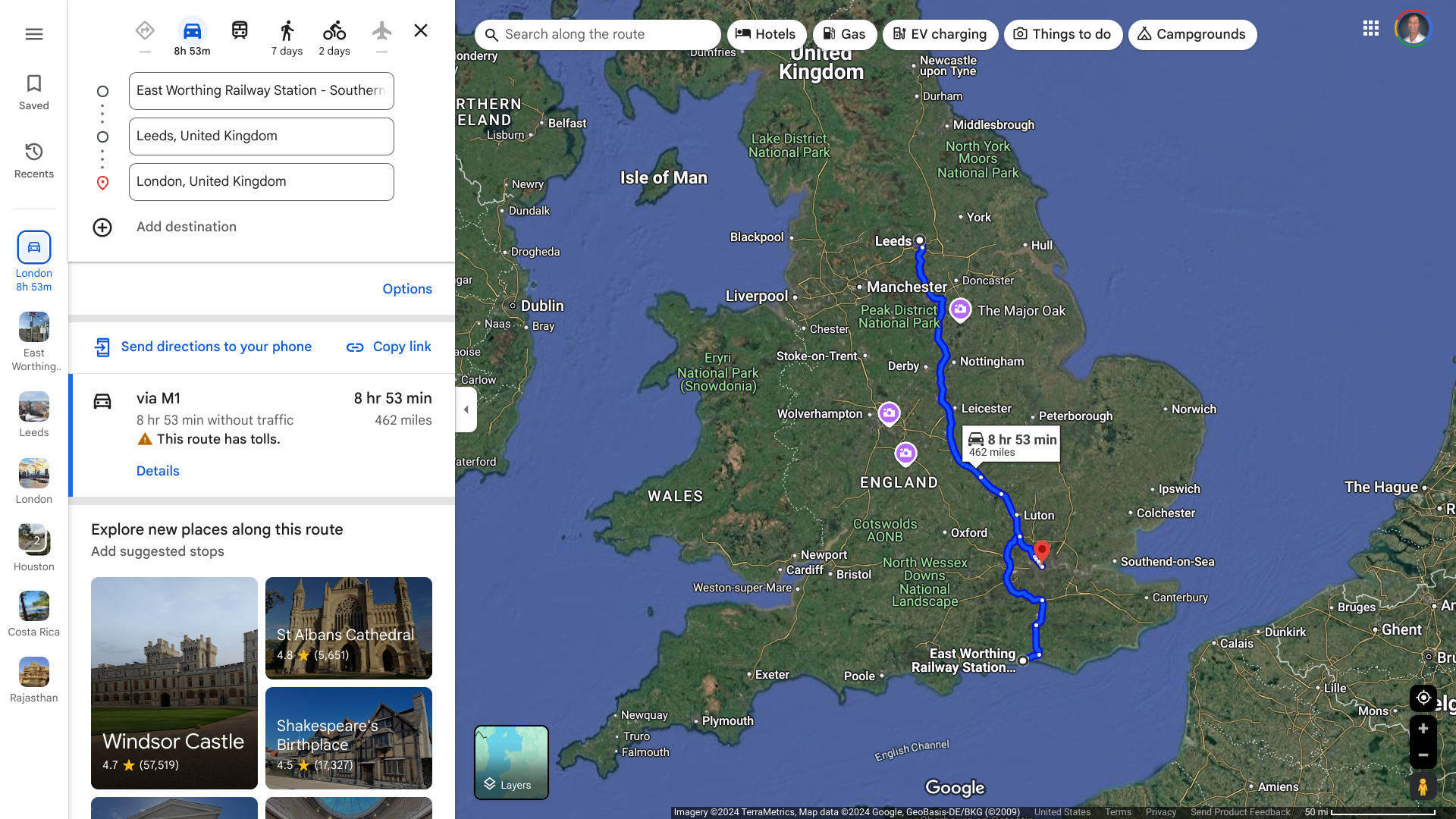
The U.S. Centers for Disease Control and Prevention (CDC) issued a Level 2 Travel Health Notice for people visiting Brazil’s Espirito Santo state due to an Oropouche virus outbreak.
Over the past weeks, Brazil has reported more than 1,300 Oropouche cases, mainly in Espirito Santo.
On December 11, 2024, the CDC stated that travelers to this southeastern Brazil state should prevent bug bites during travel to protect themselves from infection. They should also prevent bug bites for three weeks after travel to avoid possibly spreading the virus to others.
Symptoms of Oropouche include headache, fever, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light. Severe cases may result in neuroinvasive diseases such as meningitis.
Additionally, pregnant women should reconsider non-essential travel to Espírito Santo, Brazil. If travel is unavoidable, these travelers should strictly follow Oropouche prevention recommendations.
Recent reports indicate that Oropouche can be spread by sex contact.
In August 2024, an infant with microcephaly associated with Oropouche virus infection was reported in Brazil. The infant, born in June 2024, who later died at 47 days of life, had tested positive for Orepouche virus IgM.
Previously, the CDC had a Level 1 travel notice for other parts of Brazil, Bolivia, Colombia, Cuba, the Dominican Republic, Ecuador, Guyana, and Peru.
In the United States, Florida has reported over 90 travel-related Oropouche cases in 2024.
As of December 13, 2024, no Oropouche vaccine is available.
The U.S. CDC announced today that it supports the new Dosing Interval and Schedule for the Bexsero® MenB-4C Vaccine based on the Updated Recommendations of the Advisory Committee on Immunization Practices in October 2024.
On December 12, 2024, the CDC's MMWR (73(49);1124–1128) recommended MenB-4C as a 2-dose series with doses administered at intervals of 0 and 6 months for healthy adolescents and young adults aged 16–23 based on shared clinical decision-making and as a 3-dose series with doses administered at 0, 1–2, and 6 months for persons aged ≥10 years at increased risk.
This news is very relevant for teenagers and college students as real-world evidence suggests that Bexsero provides cross-protection against gonorrhea, a sexually transmitted disease.
For example, the U.K.'s Joint Committee on Vaccination and Immunisation recommended a routine targeted vaccination program in 2023 using the 4CMenB to prevent gonorrhea.

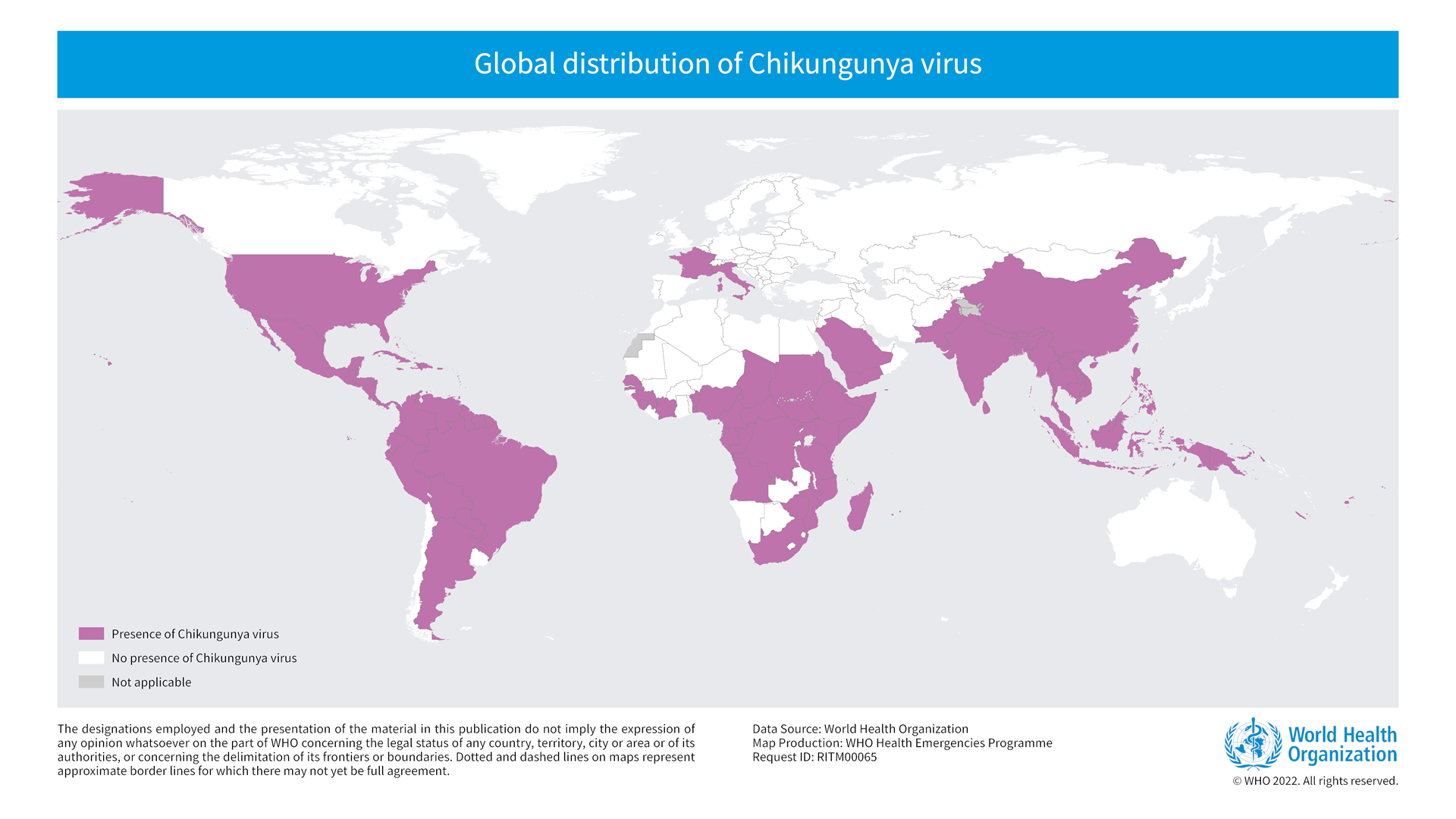
Coalition for Epidemic Preparedness Innovations (CEPI) today announced a team of scientists will soon find out the extent to which chikungunya, a mosquito-borne disease causing large outbreaks in Asia and the Region of the Americas, is also affecting countries in East Africa.
Led by the University of Oxford, the scientists will soon investigate the number of children and adults affected by chikungunya at sites in Kenya and Tanzania.
Therefore, starting in early Spring 2025, all patients, including children, presenting at ten healthcare facilities across the two countries with fever or neurological symptoms will be screened and tested for the chikungunya virus.
The new research will collect this information over the next three years to estimate the number of people with chikungunya in the region better and inform outbreak planning efforts.
Professor George Warimwe, Lead of the ACHIEVE study and Professor of Vaccinology at the University of Oxford commented in a press release on December 12, 2024, "We lack good estimates of the burden of chikungunya in East Africa, and the clinical manifestations of the disease are not well characterized, especially in children."
"This investment is an important step towards improving our understanding of chikungunya in the region, ultimately informing disease control strategies."
The first-ever approved chikungunya vaccine IXCHIQ®, was developed by Valneva SE. CEPI and partners are now working to accelerate vaccine access in outbreak-affected regions that are most at risk from the disease, such as East Africa.
In the Americas, over 416,000 chikungunya cases and 211 related fatalities have already been confirmed outbreaks in various countries in 2024.
"Chikungunya disease is a new topic for many international travelers. Educating people about the disease and its serious after effects is news to many," stated Beverly Schaefer, RPh.
"Offering a highly effective new vaccine to prevent chikungunya disease is an excellent relief to travelers heading to areas with recent outbreaks or where outbreaks are on the rise, added Schafer, travel vaccine expert at Katterman's Sand Point Pharmacy in Seattle, WA.
In the United States, IXCHIQ is commercially available at travel clinics and numerous pharmacies.
The U.S. NIH recently announced the antiviral drug tecovirimat, known commercially as TPOXX®, did not reduce the time to lesion resolution or have an effect on pain among adults with mild to moderate clade II mpox and a low risk of developing severe disease.
A planned interim analysis at 75% of the current study's target enrollment showed no difference in the time to lesion resolution between participants treated with tecovirimat compared with those who received a placebo.
This finding is based on an interim data analysis from an international clinical trial called the Study of Tecovirimat for Mpox (STOMP).
Considering these definitive findings, the study's Data Safety and Monitoring Board recommended stopping further enrollment of participants who were being randomized to tecovirimat or placebo.
At the Board's request, an additional assessment was performed. Based on the study design and available data, there was less than a 1% chance that the study would show that tecovirimat would be effective if it were to complete enrollment and follow-up.
"The initial STOMP findings provide valuable insight to inform clade II mpox medical countermeasures and underscore the critical importance of conducting well-designed randomized clinical trials during infectious disease outbreaks," said NIAID Director Jeanne Marrazzo, M.D., M.P.H., in a media release on December 10, 2024.
"Before 2022, no treatment candidate had been studied in people with mpox, and this trial is a critical step in our systematic evaluation of existing antivirals like tecovirimat while pursuing novel antivirals and antibody-based mpox therapeutics."
The Food and Drug Administration initially approved SIGA Technologies, Inc.'s Tecovirimat to treat smallpox.
Mpox is caused by a virus that spreads mainly through close contact. Two virus types, clades I and II, have been identified and are historically present in Central and West Africa.
A clade II subtype virus caused a global mpox outbreak in 2022, and the virus continues to circulate at low levels.
In 2024, the World Health Organization declared a Clade I outbreak in Central and East African countries a public health emergency of international concern.
Travel-related cases of clade I mpox have been reported internationally, and the first reported case in the United States was diagnosed on November 15, 2024.
The U.S. CDC has issued a Level 2 travel advisory regarding the Mpox outbreak.

According to NBC News, on December 11, 2024, the U.S. administration informed the media that there are no active plans to authorize the distribution of avian influenza pandemic (bird flu) vaccines.
This news is similar to what the U.S. Centers for Disease Control and Prevention stated on June 27, 2024, when it confirmed its avian vaccination program was inactive.
Previously, the U.S. National Influenza Vaccine Modernization Strategy and the American Pandemic Preparedness Plan outlined priorities for avian influenza vaccines.
Over the past few years, the U.S. FDA has approved one vaccine (CSL Seqirus Inc. Audenz™), and the U.S. government has funded various avian influenza vaccine initiatives.
In Europe, CSL Seqirus announced on June 11, 2024, that it would provide 665,000 pre-pandemic (zoonotic) vaccines to fifteen E.U. and EEA Member States. Seqirus UK Ltd has an EU-wide modified marketing authorization for this avian influenza vaccine for use in adults.

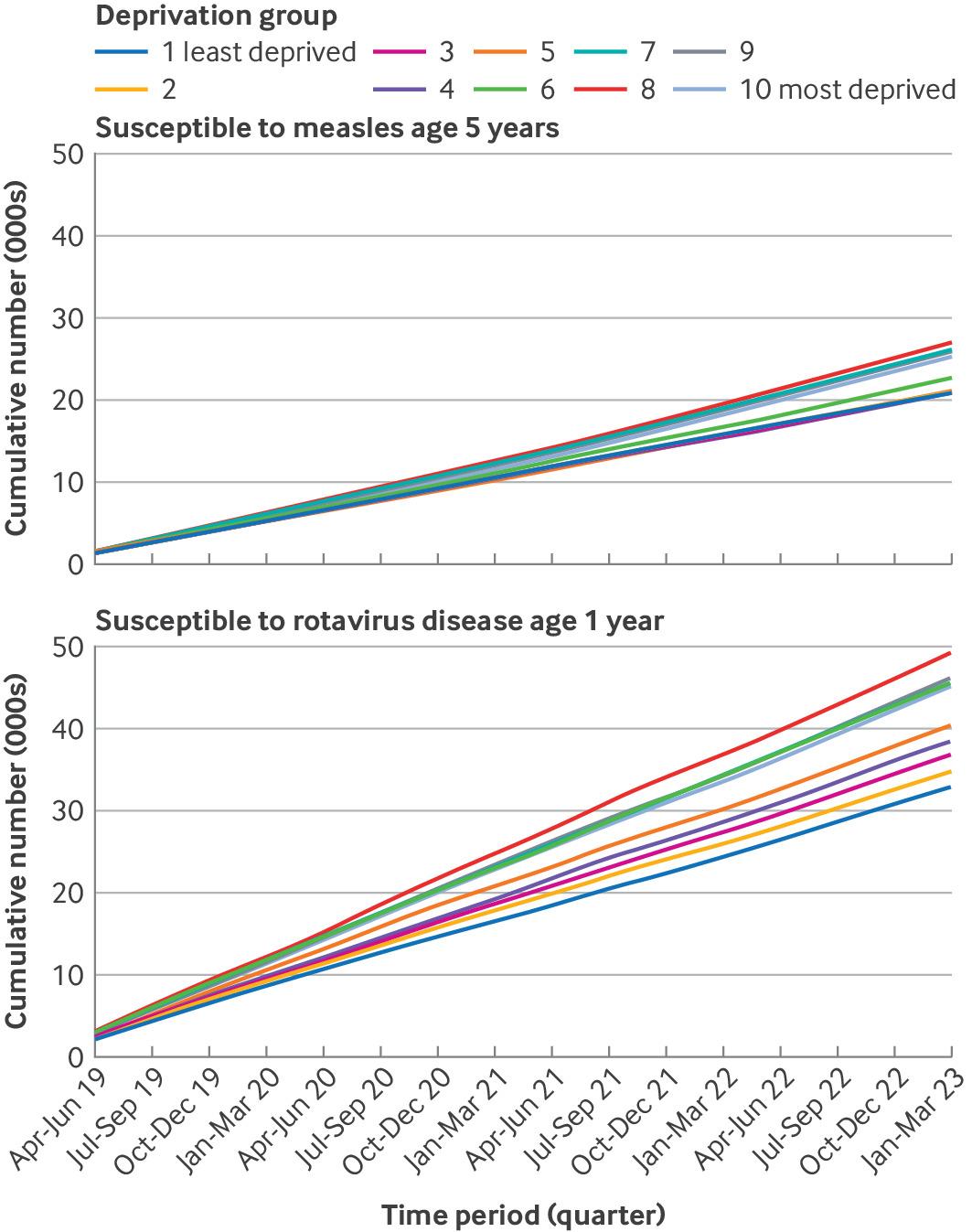
According to a study published in The BMJ, the rate of essential childhood vaccinations has recently decreased in the U.K. The vaccination schedule in England protects children against 15 vaccine-preventable diseases, and vaccines are administered from ages 8 weeks to 14 years.
Published on December 11, 2024, these researchers analyzed vaccination rates at general practices for MMR, rotavirus, pneumococcal conjugate booster, and a six-in-one shot that includes diphtheria, tetanus, and polio vaccinations.
For example, the estimated cumulative number of 5-year-olds susceptible to measles infection over the study period increased 15-fold in the least deprived group.
This study revealed that vaccination rates in England have declined steadily since 2013, with few in the routine vaccination schedule. For all childhood vaccinations studied, the uptake rates in England did not exceed the WHO recommended threshold of 95% in the more deprived populations.
As of December 12, 2024, the U.S. CDC says that when visiting the U.K., one should be aware of current health issues such as measles.
Novavax, Inc. announced that the first participants have been dosed in its COVID-19-Influenza Combination (CIC) and stand-alone seasonal influenza Phase 3 clinical trial.
The trial will evaluate the immunogenicity and safety of the CIC and stand-alone seasonal influenza vaccine candidates compared to Novavax's updated 2024-2025 COVID-19 vaccine (NVX-CoV2705) and a licensed seasonal influenza vaccine comparator in adults aged 65 and older.
"A combination vaccine for two vaccine-preventable diseases is an important step forward for public health, and the trial start is a key step in advancing our late-stage pipeline, which we plan to progress through strategic partnerships," said Ruxandra Draghia-Akli, MD, PhD, Executive Vice President, Head of Research and Development, Novavax, in a press release on December 10, 2024.
"Our goal is to get these assets to market as soon as possible, and we will work with the U.S. FDA to assess the possibility of an accelerated approval pathway."
The Company is working with the U.S. FDA to determine the potential of the current CIC and stand-alone influenza trial to support accelerated approval.
As of December 2024, Novavax vaccines are available at pharmacies in the U.S. and in various countries.
