Search API
The World Health Organization (WHO) today announced that the current cholera outbreak in the Republic of South Sudan has recorded 21,000 cases and 367 deaths.
As of January 22, 2025, this cholera outbreak, which the government declared in October 2024, has been reported across seven states. The leading counties in this East African country are Rubkona, with 47% of total cases, followed by Juba, at around 10%.
The government launched oral cholera vaccination (OCV) campaigns in four high-risk counties in January 2025 to address the rising number of cholera cases.
With support from Gavi, the Vaccine Alliance, around 4 million vaccine doses have been approved, and around 910,000 doses have been administered.
The WHO previously prequalified several OCVs.
The WHO has recorded seven cholera pandemics over the past two centuries. The current (7th) cholera epidemic is considered to have started in 1961 and continues in forty-five countries in 2025, with a case fatality rate of 0.6%.
In 2025, cholera vaccination is recommended when visiting cholera outbreaks. Additionally, due to outbreaks in the region, the U.S. CDC recommends protecting visitors to South Sudan against measles and polio.
Travel vaccines and OCVs are offered at travel clinics and pharmacies in the U.S.

Over the past year, Quebec has faced measles outbreaks in various cities. The first outbreak of 2024 led to 51 cases.
Since December 2024 and as of January 21, 2025, Quebec's Health Ministry has confirmed 13 measles cases as part of the second outbreak. These measles cases are in Laurentides (7), Montréal, and Laval.
Certain places frequented by recent measles cases have been identified. People must isolate themselves if they are not protected against measles. Update on January 22, 2025, this list has more information as part of the ongoing investigation.
Quebec is not alone in Canada, as the country reported 147 measles cases, the most in about a decade.
As of today, the U.S. CDC has not issued a travel advisory for Quebec's measles outbreak(s). Each year, millions of international visitors travel to this area of Canada.
In 2024, the CDC confirmed several measles outbreaks in the U.S.
The World Health Organization's Disease Outbreak News recently reported a fatal case of Chapare hemorrhagic fever (CHHF) from the La Paz Department in the Plurinational State of Bolivia.
As of January 20, 2025, no secondary cases have been reported.
The WHO defines Chapare hemorrhagic fever as an acute viral illness caused by the Chapare virus. The rodent-borne Chapare virus is an Arenavirus that can cause hemorrhagic fevers like Ebolaviruses.
Initially identified in Cochabamba in 2003, five documented outbreaks have occurred within Bolivia.
The most recent outbreak occurred in 2024, with one laboratory-confirmed case within the La Paz Department. This area in Bolivia, which has a population of about 3 million, is a neighbor of Peru.
As of January 22, 2025, the WHO says there is no significant risk of the disease spreading internationally. Person-to-person transmission of the Chapare virus is possible but remains rare in the general population.
The U.S. CDC says CHHF is a rare, deadly viral disease. About 20% to 60% of people with the disease die.
Furthermore, there are no treatments or preventive vaccines available for CHHF.
When visiting Bolivia in 2025, the CDC recommends several travel vaccinations, such as chikungunya and yellow fever. These vaccines are offered at many travel clinics and pharmacies in the U.S.
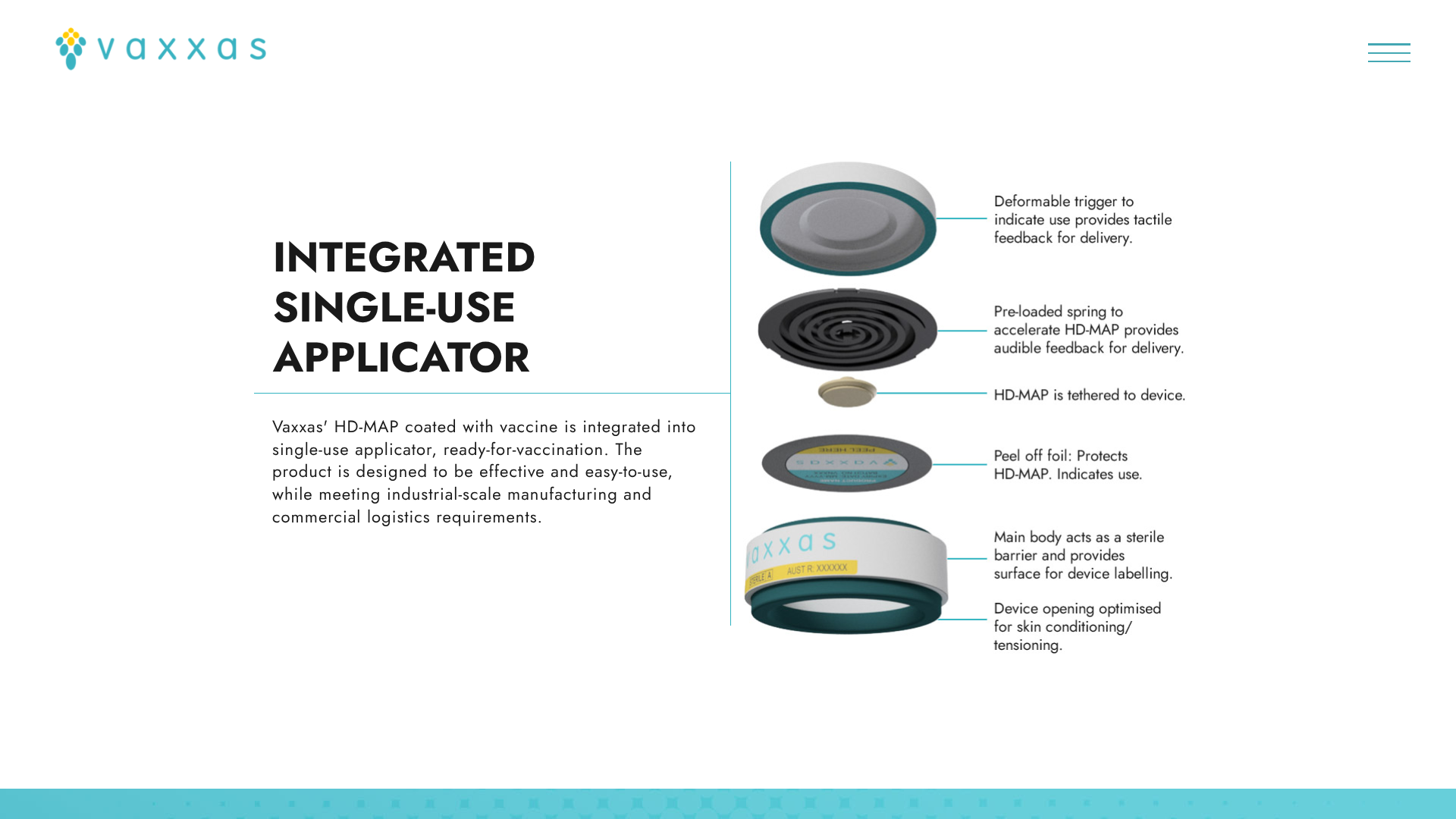
Vaxxas today announced that the Coalition for Epidemic Preparedness Innovations (CEPI) approved the progression of a $4.8 million program to develop heat-stable, dried-formulation mRNA vaccines delivered using Vaxxas’ needle-free high-density microarray patch (HD-MAP).
The Vaxxas HD-MAP is comprised of thousands of microscopic projections molded into a small patch. Each microprojection is coated with a small dose of vaccine in a dried formulation.
Announced on January 22, 2025, Vaxxas will partner with SK bioscience in this next phase of the program, advancing the company’s mRNA vaccine for Japanese Encephalitis Virus (JEV) on Vaxxas’ HD-MAP towards a Phase I clinical study.
In late 2024, several JEV cases were confirmed in various Asian and Western Pacific Ocean countries.
Vaxxas expects the development work performed with the JEV vaccine candidate to be transferrable across all mRNA vaccine antigens delivered by LNPs, providing a platform approach that can be advanced to human trials.
David L. Hoey Vaxxas, CEO and President, commented in a press release, "With compelling proof-of-concept results in hand, we’re excited to have CEPI’s commitment to advance to the next stage of development."
"We’re equally excited to be working with SK bioscience and its JEV mRNA vaccine on this program to realize the promise of our HD-MAP technology to move the world closer to a commercially available, thermostable patch-based mRNA vaccine.”
This program was funded by CEPI in 2023 as part of its aim to improve the thermostability, and therefore equitable access, of mRNA vaccines.
This program is Vaxxas’ second collaboration with SK bioscience. The companies are also working on a program funded by Wellcome to advance the development of an HD-MAP/Typhoid conjugate vaccine candidate.
The reemergence of yellow fever in the Brazilian state of São Paulo over the past 23 years has highlighted the need to be fully immunized before visiting endemic areas in 2025.
The São Paulo State Health Department recently confirmed the first human yellow fever case in January 2025.
According to a study published by Rev Bras Epidemiol in December 2024, five yellow fever outbreaks from 2000 to 2023 led to 679 human cases. Epizootic surveillance actions in non-human primates intensified in 2017 when the virus circulated in areas without vaccine recommendations in the state.
A previous study found the metropolitan region of São Paulo city YF outbreak during 2017–2018 revealed that 36 deaths were due to three genetic variants of sylvatic YFV that belong to the South American I genotype and that were related to viruses previously isolated from other locations in Brazil (Minas Gerais, Espírito Santo, Bahia, and Rio de Janeiro states).
According to these researchers, each variant represented an independent YF virus introduction into Sao Paulo.
"The recently confirmed case of yellow fever infection in São Paulo reinforces the importance of vaccination before traveling to many parts of Brazil, including popular urban destinations," commented Jeri Beales, MSN, RN.
"The 2017 yellow fever outbreak in Brazil marked an important change in CDC yellow fever recommendation for Brazil-bound travelers, including major cities like São Paulo, Rio de Janeiro, Curibita, and Salvador."
"Ideally, the yellow fever vaccine is given at least 10 days before arrival to a risk area, and only clinics certified with CDC can provide the vaccine," added Beales, who leads Destination Health Clinic, a Boston-area travel health provider specializing in health education and vaccination for international travelers.
As of January 21, 2025, the U.S. CDC and the U.K. Health Security Agency recommends international travelers visit a travel clinic or pharmacy to discuss travel vaccine options about one month before visiting Brazil in 2025. This year, about 8 million people may visit Sao Paulo.
According to the CDC, the yellow fever vaccine is recommended for many destinations in Brazil, including Sao Paulo, but may not required for entry.
Note: This Vax-Before-Travel news article was updated with related insight on Jan. 22, 2025.

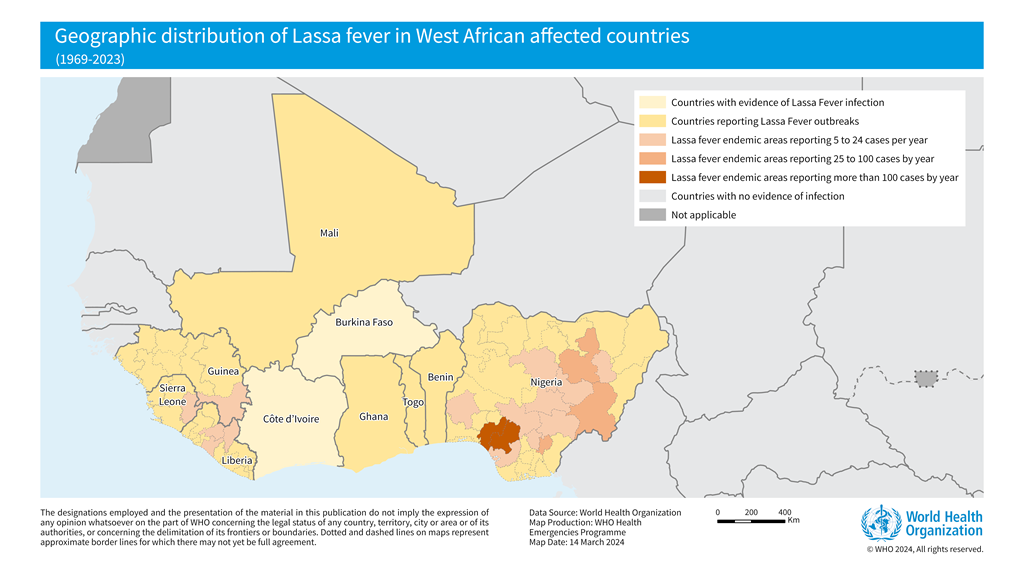
The World Health Organization (WHO) recently confirmed that it closely supports Lassa fever-endemic countries in West Africa, such as Benin, Ghana, Guinea, Liberia, Mali, Nigeria, and Sierra Leone.
As of January 19, 2025, another Lassa fever outbreak, a rare, often fatal, viral hemorrhagic fever, was confirmed in the Federal Republic of Nigeria.
The Nigeria Centre for Disease Control and Prevention (NCDC) confirmed 54 cases of Lassa fever in Ondo, Edo, and Bauchi from December 30, 2024, to January 5, 2025. The NCDC also reported 10 related fatalities, resulting in a Case Fatality Rate of 18.5%.
The NCDC continues to address the ongoing Lassa fever outbreak, which coincides with the peak season. Lassa virus was first identified in 1969 in Nigeria.
In 2024, Nigeria recorded over 1,187 confirmed cases across 28 states.
In December 2024, Dr. Jide Idris, Director-General of the NCDC, announced that the Emergency Operations Centre had been activated for Lassa fever, and the risk assessment was classified as high.
In the United States, the Iowa Department of Health and Human Services confirmed a resident died from Lassa fever in October 2024. There have been eight travel-associated cases of Lassa fever in the U.S. in the past 55 years.
As of January 21, 2025, Lassa fever vaccine candidates have not been approved for human use.
In 2024, many European countries detected poliovirus in wastewater systems, signaling the once-eradicated disease's potential resurgence.
According to the Global Polio Eradication Initiative (GPEI), poliovirus continues to be detected in Bonn, Nordrhein-Westfalen, Sachsen, and Bayern, Germany. As of January 15, 2025, eleven circulating vaccine-derived poliovirus type 2-positive environmental samples were collected in November and December 2024.
This pathogen is not the wild poliovirus type but originates from the oral polio vaccine, which contains weakened but live polioviruses. The weakened vaccine viruses can be excreted and spread by vaccinated people.
Germany's last case of wild poliovirus was recorded in 1990.
The GPEI has confirmed that various countries have also reported cases of wild polio, vaccine-derived poliovirus type 2, and circulating vaccine-derived poliovirus type 1.
On January 15, 2025, the U.S. Centers for Disease Control and Prevention (CDC) identified 39 countries at-risk for polio. This CDC list does not include Germany or the United Kingdom.
Last year, Dr Hans Henri P. Kluge, WHO Regional Director for Europe, commented in a press release, “For over 20 years, sustained efforts to achieve high vaccination coverage, quality surveillance, and rapid outbreak response have prevented the virus from re-establishing in this Region. These efforts must be commended, but also intensified as challenges to our collective defense against this virus increase.”
The CDC and the WHO recommend that all travelers to polio-affected areas in 2025 be fully vaccinated, and some people may qualify for a polio vaccine booster dose.

Moderna, Inc. recently announced ongoing support from the U.S. Department of Health and Human Services (HHS) to accelerate the development of mRNA-based pandemic influenza vaccines.
In 2023, Moderna initiated a Phase 1/2 clinical study to generate safety and immunogenicity data for an investigational pandemic influenza vaccine (mRNA-1018). The study included vaccine candidates against H5 and H7 avian influenza viruses.
Announced on January 17, 2025, the $590 million award was made through the Rapid Response Partnership Vehicle Consortium with funding from the Biomedical Advanced Research and Development Authority.
The project will support the late-stage development and licensure of pre-pandemic mRNA-based vaccines. The HHS-Moderna agreement will also expand clinical studies for up to five additional subtypes of pandemic influenza.
As of January 21, 2025, the Phase 1/2 results have not been released; however, Moderna is preparing to advance mRNA-1018 into Phase 3 clinical study.
Today's funding follows the $176 million the U.S. government awarded Moderna in July 2024.
The U.S. and European governments have invested in developing avian and pandemic influenza vaccines for years, and the U.S. Has previously approved one vaccine.
The UK government says pandemic influenza viruses are characterized by their tendency to change rapidly, their ability to spread quickly, and the routes of transmission, which contribute to the difficulty in containing an infectious global outbreak.
Influenza A viruses are most likely to cause influenza pandemics due to an extensive reservoir of these viruses in animal populations, particularly avian and swine, to which humans have no immunity.
Compared to seasonal influenza, population immunity to the new influenza A virus is nonexistent or sufficiently low to facilitate rapid person-to-person transmission and increase the severity of illness among those infected. This is generally associated with higher rates of disease and death.
Furthermore, annual 'flu=shots' are not expected to protect people from pandemic influenza viruses.
On December 11, 2024, the U.S. administration informed the media that there are no active plans to authorize the distribution of avian influenza (bird flu) vaccines.