UTI Appointment Request Response
As you may be aware, the Uromune MV140 vaccine is not FDA-approved for use in the United States as of October 2025. However, this oral spray UTI vaccine is available 'off-label' in various countries.
- In Canada, Red Leaf Medical has voluntarily withdrawn the application to address deficiencies in the submission identified by Health Canada.
- In Mexico, the Benavides pharmacy website lists Uromune, but inventory has been limited, and you need a local doctor's prescription. Several other pharmacies in Mexico also offer Uromune.
- In England, clincis near London offers the UTI vaccine, such as The Urology Partnership at The Forbury Clinic.
- In Portugal, Dr. Ricardo Pereira e Silva and collaborators the Hospital de Santa Maria – Lisbon. Online interview link.
- According to the manufacturer, Uromune is not offered in Spain.
- In the Dominican Republic, here is the contact for Luis E. Betances & Co. SAS.
- Across the Pacific, this clinic at Mount Elizabeth Novena Hospital and Uro Health in Singapore offer UTI services.
- Uromune is not yet approved in Denmark, France, Germany, India or Japan.
Additionally, here is a link to UTI information about:
- Uro-Vaxom® (OM-89) whose active ingredient is the bacterial lysate acts as a vaccine. Since its launch, millions of patients have been treated with OM-89.
- Blujepa is approved for the treatment of female adults (≥40 kg) and pediatric patients with uUTIs.
- Pivya (pivmecillinam) tablets for Uncomplicated UTIs.
- RECCE 327 is an intravenous and topical UTI therapy.
- Complicated urinary tract infections (cUTIs) pivotal Phase 3 clinical trial announced positive data. If approved, tebipenem HBr could be the first oral carbapenem antibiotic for patients with cUTIs.
- The BCG vaccine is used off-lable for various bacteria related infections.
- LBP-EC01 is a bacteriophage cocktail engineered with a CRISPR-Cas3 construct targeting the E. coli genome, treating uncomplicated UTIs.
- UTI clinical trials in the USA.
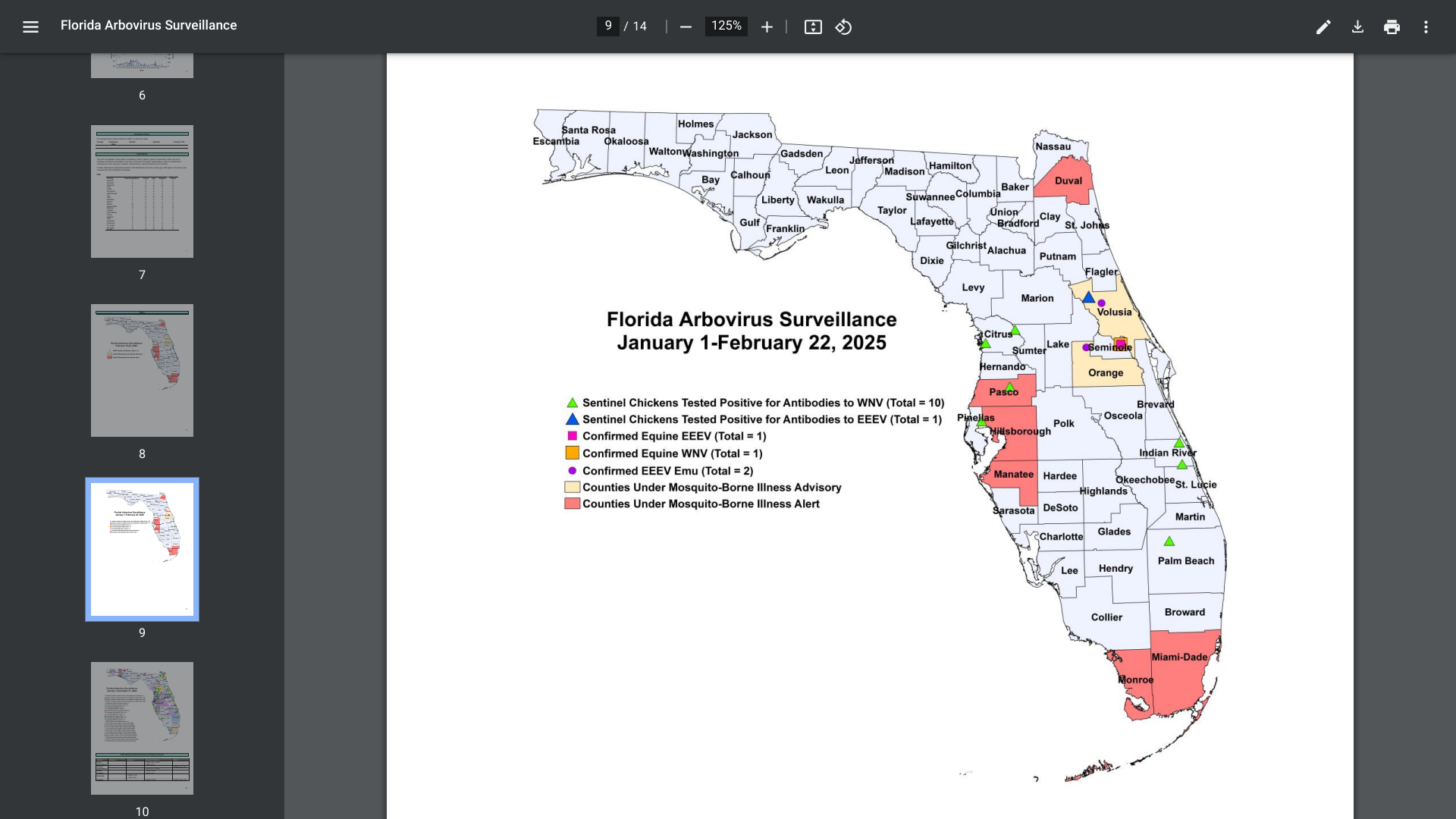
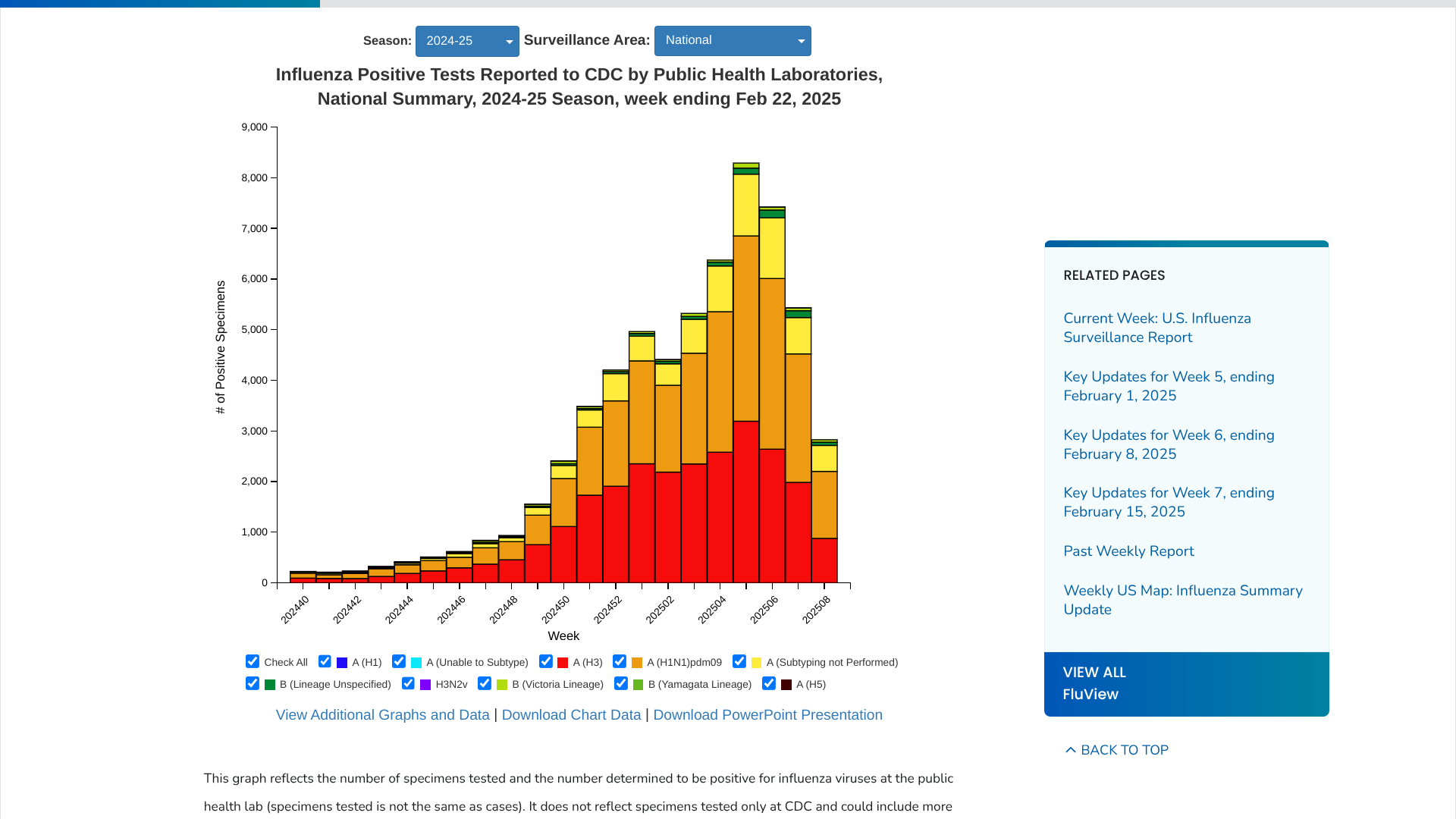
In addition to UTIs, Vax-Before-Travel publishes breaking news and travel vaccine information.
By the way, I am not a healthcare provider, and this publicly available information is not health advice. If you have questions, please contact your healthcare provider.
Thank you,
Don
Publisher, Vax-Before-Trave LLC