Search API
Following a disheartening 2024, the Islamic Republic of Pakistan recently reported three new wild poliovirus type 1 (WPV1) patients.
As of January 22, 2025, the Global Polio Eradication Initiative (GPEI) confirmed that these WPV1 cases were reported in Khyber Pakhtunkhwa and Sindh.
Last year, 73 WPV1 patients were confirmed.
Of these, 27 were from Balochistan, 22 from Khyber Pakhtunkhwa, 22 from Sindh, and one from Punjab and Islamabad.
Pakistan's National Emergency Operations Centre recently wrote, 'Considering the intense polio outbreak, parents must ensure vaccination for all their children under five to protect them.'
Polio remains a vaccine-preventable but paralyzing disease that has no cure.
There are three serotypes of wild poliovirus. Immunity to one serotype does not confer immunity to the other two.
Type 2 wild poliovirus was declared eradicated in 2015, and type 3 was declared eradicated in 2019. Only type 1 wild poliovirus remains in 2025.
In late December 2024, the GPEI wrote, 'This resurgence of the virus underscores that there is no room for error in an eradication program. To stop wild poliovirus in its last frontier of Pakistan and Afghanistan, the program is working to strengthen cross-border coordination and deliver a broader range of health interventions alongside polio vaccines.'
In 2023, Pakistan reported over 900,000 visitors, a new record for international tourist arrivals.
To alert international travelers of this health risk, the U.S. CDC reissued a Global Polio Travel Health Advisory on January 14, 2025. The CDC says adults who previously completed the full, routine polio vaccine series before traveling to any destination listed may receive a single, lifetime booster dose of polio vaccine.
In the U.S., polio vaccines are offered at travel clinics and pharmacies in 2025.
The World Health Organization (WHO) today announced that the current cholera outbreak in the Republic of South Sudan has recorded 21,000 cases and 367 deaths.
As of January 22, 2025, this cholera outbreak, which the government declared in October 2024, has been reported across seven states. The leading counties in this East African country are Rubkona, with 47% of total cases, followed by Juba, at around 10%.
The government launched oral cholera vaccination (OCV) campaigns in four high-risk counties in January 2025 to address the rising number of cholera cases.
With support from Gavi, the Vaccine Alliance, around 4 million vaccine doses have been approved, and around 910,000 doses have been administered.
The WHO previously prequalified several OCVs.
The WHO has recorded seven cholera pandemics over the past two centuries. The current (7th) cholera epidemic is considered to have started in 1961 and continues in forty-five countries in 2025, with a case fatality rate of 0.6%.
In 2025, cholera vaccination is recommended when visiting cholera outbreaks. Additionally, due to outbreaks in the region, the U.S. CDC recommends protecting visitors to South Sudan against measles and polio.
Travel vaccines and OCVs are offered at travel clinics and pharmacies in the U.S.

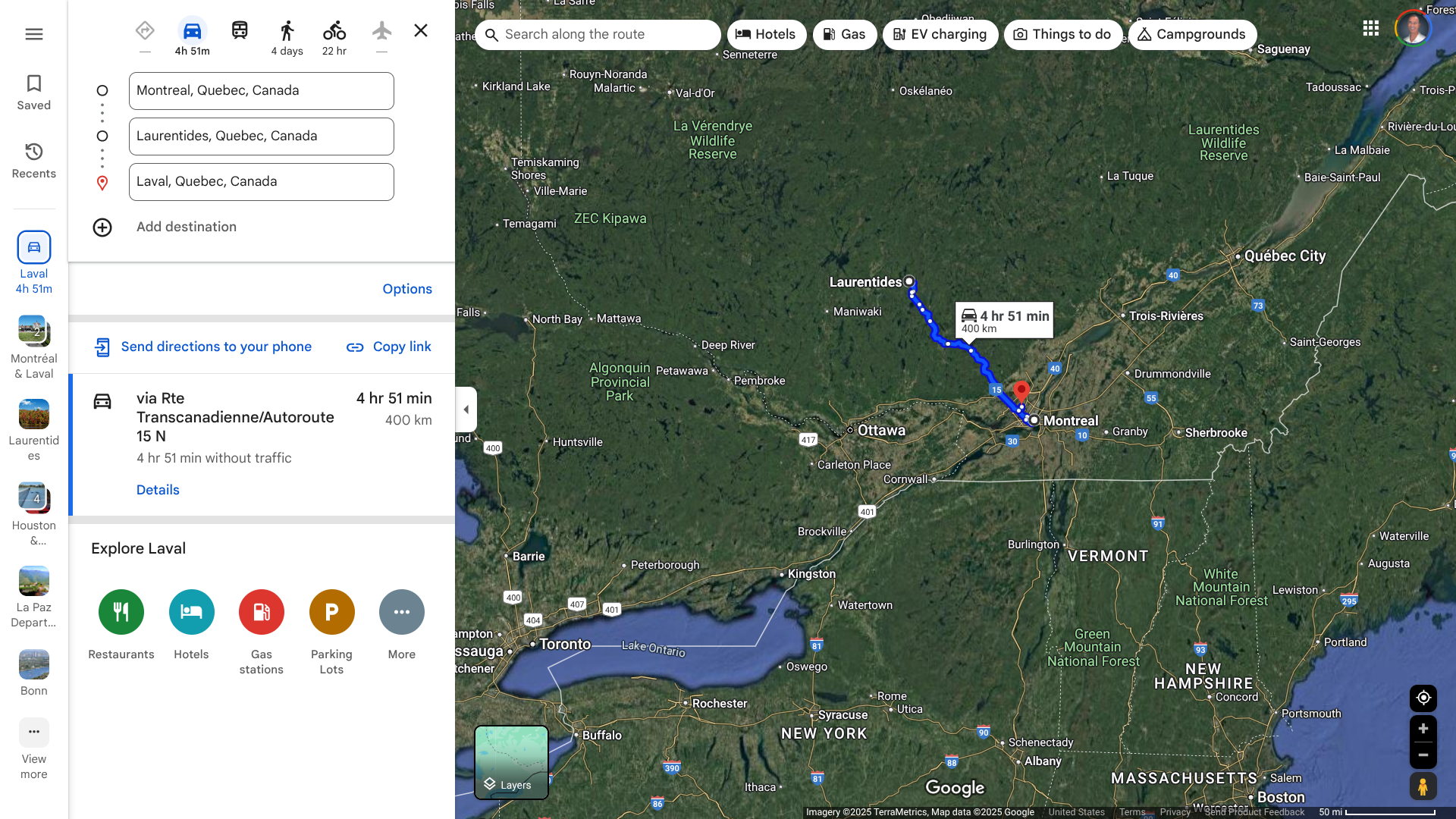
Over the past year, Quebec has faced measles outbreaks in various cities. The first outbreak of 2024 led to 51 cases.
Since December 2024 and as of January 21, 2025, Quebec's Health Ministry has confirmed 13 measles cases as part of the second outbreak. These measles cases are in Laurentides (7), Montréal, and Laval.
Certain places frequented by recent measles cases have been identified. People must isolate themselves if they are not protected against measles. Update on January 22, 2025, this list has more information as part of the ongoing investigation.
Quebec is not alone in Canada, as the country reported 147 measles cases, the most in about a decade.
As of today, the U.S. CDC has not issued a travel advisory for Quebec's measles outbreak(s). Each year, millions of international visitors travel to this area of Canada.
In 2024, the CDC confirmed several measles outbreaks in the U.S.
The World Health Organization's Disease Outbreak News recently reported a fatal case of Chapare hemorrhagic fever (CHHF) from the La Paz Department in the Plurinational State of Bolivia.
As of January 20, 2025, no secondary cases have been reported.
The WHO defines Chapare hemorrhagic fever as an acute viral illness caused by the Chapare virus. The rodent-borne Chapare virus is an Arenavirus that can cause hemorrhagic fevers like Ebolaviruses.
Initially identified in Cochabamba in 2003, five documented outbreaks have occurred within Bolivia.
The most recent outbreak occurred in 2024, with one laboratory-confirmed case within the La Paz Department. This area in Bolivia, which has a population of about 3 million, is a neighbor of Peru.
As of January 22, 2025, the WHO says there is no significant risk of the disease spreading internationally. Person-to-person transmission of the Chapare virus is possible but remains rare in the general population.
The U.S. CDC says CHHF is a rare, deadly viral disease. About 20% to 60% of people with the disease die.
Furthermore, there are no treatments or preventive vaccines available for CHHF.
When visiting Bolivia in 2025, the CDC recommends several travel vaccinations, such as chikungunya and yellow fever. These vaccines are offered at many travel clinics and pharmacies in the U.S.
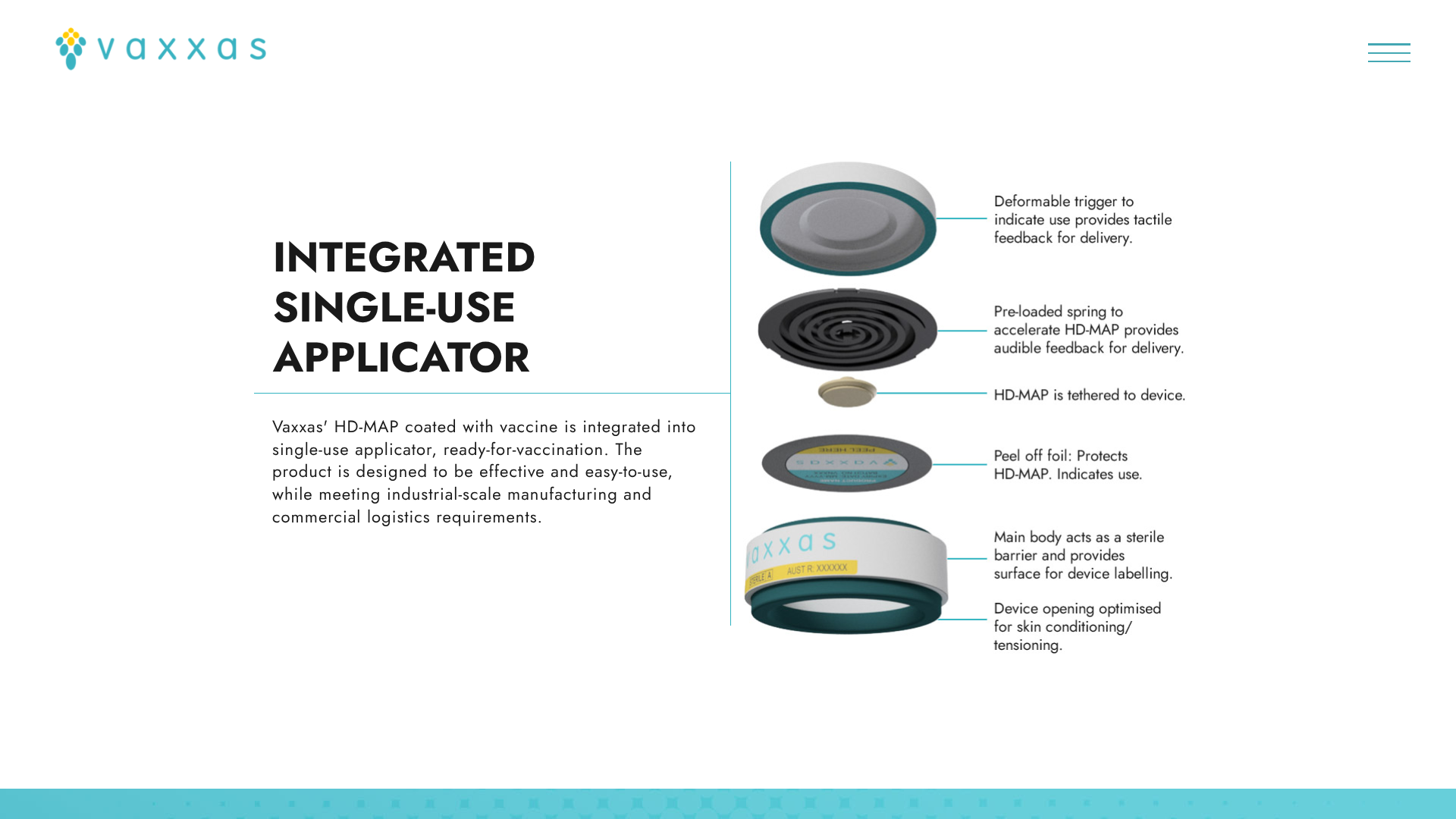
Vaxxas today announced that the Coalition for Epidemic Preparedness Innovations (CEPI) approved the progression of a $4.8 million program to develop heat-stable, dried-formulation mRNA vaccines delivered using Vaxxas’ needle-free high-density microarray patch (HD-MAP).
The Vaxxas HD-MAP is comprised of thousands of microscopic projections molded into a small patch. Each microprojection is coated with a small dose of vaccine in a dried formulation.
Announced on January 22, 2025, Vaxxas will partner with SK bioscience in this next phase of the program, advancing the company’s mRNA vaccine for Japanese Encephalitis Virus (JEV) on Vaxxas’ HD-MAP towards a Phase I clinical study.
In late 2024, several JEV cases were confirmed in various Asian and Western Pacific Ocean countries.
Vaxxas expects the development work performed with the JEV vaccine candidate to be transferrable across all mRNA vaccine antigens delivered by LNPs, providing a platform approach that can be advanced to human trials.
David L. Hoey Vaxxas, CEO and President, commented in a press release, "With compelling proof-of-concept results in hand, we’re excited to have CEPI’s commitment to advance to the next stage of development."
"We’re equally excited to be working with SK bioscience and its JEV mRNA vaccine on this program to realize the promise of our HD-MAP technology to move the world closer to a commercially available, thermostable patch-based mRNA vaccine.”
This program was funded by CEPI in 2023 as part of its aim to improve the thermostability, and therefore equitable access, of mRNA vaccines.
This program is Vaxxas’ second collaboration with SK bioscience. The companies are also working on a program funded by Wellcome to advance the development of an HD-MAP/Typhoid conjugate vaccine candidate.
The reemergence of yellow fever in the Brazilian state of São Paulo over the past 23 years has highlighted the need to be fully immunized before visiting endemic areas in 2025.
The São Paulo State Health Department recently confirmed the first human yellow fever case in January 2025.
According to a study published by Rev Bras Epidemiol in December 2024, five yellow fever outbreaks from 2000 to 2023 led to 679 human cases. Epizootic surveillance actions in non-human primates intensified in 2017 when the virus circulated in areas without vaccine recommendations in the state.
A previous study found the metropolitan region of São Paulo city YF outbreak during 2017–2018 revealed that 36 deaths were due to three genetic variants of sylvatic YFV that belong to the South American I genotype and that were related to viruses previously isolated from other locations in Brazil (Minas Gerais, Espírito Santo, Bahia, and Rio de Janeiro states).
According to these researchers, each variant represented an independent YF virus introduction into Sao Paulo.
"The recently confirmed case of yellow fever infection in São Paulo reinforces the importance of vaccination before traveling to many parts of Brazil, including popular urban destinations," commented Jeri Beales, MSN, RN.
"The 2017 yellow fever outbreak in Brazil marked an important change in CDC yellow fever recommendation for Brazil-bound travelers, including major cities like São Paulo, Rio de Janeiro, Curibita, and Salvador."
"Ideally, the yellow fever vaccine is given at least 10 days before arrival to a risk area, and only clinics certified with CDC can provide the vaccine," added Beales, who leads Destination Health Clinic, a Boston-area travel health provider specializing in health education and vaccination for international travelers.
As of January 21, 2025, the U.S. CDC and the U.K. Health Security Agency recommends international travelers visit a travel clinic or pharmacy to discuss travel vaccine options about one month before visiting Brazil in 2025. This year, about 8 million people may visit Sao Paulo.
According to the CDC, the yellow fever vaccine is recommended for many destinations in Brazil, including Sao Paulo, but may not required for entry.
Note: This Vax-Before-Travel news article was updated with related insight on Jan. 22, 2025.